Abstract
Membranous nephropathy (MN) is defined as disease entity characterized by thickening of the glomerular basement membranes due to subepithelial (SE) deposition of immune complexes. It is typically classified into primary MN (70%) when there is no disease association, and secondary MN (30%) when there is an underlying disease association such as lupus, malignancy, infections or drugs. Phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A (THSD7A) are target antigens in 70% and 1–5% of primary MN, respectively. The antigens in the remaining MN were not known. Recently, multiple novel proteins/target antigens have been identified in MN. These include exostosin 1/2, neural epidermal growth-like 1 protein, semaphorin 3B, protocadherin 7 and neural cell adhesion molecule 1. Some of these antigens are present in the setting of primary MN, some in secondary MN and some in both, thus blurring the lines between primary and secondary MN. Preliminary studies show that each of the new antigen-associated MN has distinct clinical, kidney biopsy findings and outcome data. We propose that each new protein/antigen-associated MN is a specific disease that results in the common MN pattern of injury characterized by thickened glomerular basement membrane (GBM) with or without spikes or pinholes on light microscopy, granular immunoglobulin G with or without complement 3 on immunofluorescence microscopy and SE electron-dense deposits on electron microscopy. In other words, MN is truly only a pattern of injury resulting from specific diseases that cause deposition of SE immune deposits along the GBM. It is of paramount importance to ascertain the specific disease entity causing the MN pattern not only for precise diagnosis and management, but also for future studies on these newly described diseases.
Keywords: exostosin 1/2, membranous nephropathy, neural cell adhesion molecule 1, neural epidermal growth factor like-1 protein, protocadherin 7, semaphorin 3B
INTRODUCTION
Membranous nephropathy (MN) is an entity defined by the presence of immunoglobulin (Ig) deposits along the subepithelial (SE) aspect of the glomerular basement membrane (GBM). MN occurs more often in the adult population and is characterized by the development of nephrotic syndrome. The kidney biopsy in MN shows thickened GBM often with development of spikes and pinholes that are best seen on periodic acid–Schiff and silver-stained sections, granular polyclonal IgG along the GBM and SE electron-dense deposits. Complement activation is often present as evidenced by deposition of complement 3 (C3) along the GBM. The SE electron-dense deposits over time are incorporated into the GBM. Thus, based on the location and characteristics of the deposits on electron microscopy (EM), MN is staged from I through IV, which is helpful in indicating the chronicity of MN lesion [1].
Based on the clinical presentation of nephrotic syndrome and the biopsy findings, MN is thought of as a specific pathologic disease [2]. Historically, MN is typically divided into primary MN when there is no underlying disease association, and secondary MN when there is an underlying disease association such as autoimmune disease (most commonly lupus), malignancy, infection or temporal association with the use of certain drugs [3]. Until recently, the antigens responsible for MN were not known. The finding of neutral endopeptidase as the responsible antigen in a rare subset of patients with alloimmune antenatal MN paved the way for the detection of other intrinsic podocyte antigens [4]. Subsequently, in 2009 and 2014, phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A (THSD7A) were identified as two target antigens in MN: PLA2R was primarily the target antigen in a majority of primary MN whereas THSD7A was present in primary MN but also in secondary MN associated with a malignancy [5, 6]. PLA2R and THSD7A antigens account for ∼50–60% of target antigens in all MN (including primary and secondary MN).
Recently, specific proteins/putative antigens associated with lupus/other autoimmune diseases and new antigens have been identified in MN. These ‘new’ antigens include exostosin 1/2 (EXT1/2), neural epidermal growth-like 1 protein (NELL1), semaphorin 3B (SEMA3B), protocadherin 7 (PCDH7) and neural cell adhesion molecule 1 (NCAM1) [7–12]. Three more antigens related to specific secondary MN disease associations are under active investigation (unpublished data). Some of the new antigens are present in primary MN, others are present in secondary MN and some in both. Thus, the identification of the new antigens has blurred the lines between primary and secondary MN. On careful study of MN associated with the ‘new’ antigens, we find that they have distinct clinical and pathologic findings suggesting that each antigen-associated MN is a specific disease entity.
Traditionally, glomerulonephritis (GN) was classified based on the light microscopy (LM) pattern of injury: crescentic GN, membranoproliferative GN (MPGN), diffuse proliferative GN, etc. As progress in identifying the varying etiologies associated with a specific pattern and understanding the pathophysiology of development of the specific pattern was achieved, the classification was modified to an etiology-based classification [13]. For example, crescentic GN is now classified into ANCA-associated GN [14], anti-GBM associated GN and immune complex or complement (C3G)-associated GN [15]; MPGN is classified into immune-complex associated MPGN resulting from either autoimmune diseases, chronic infections, monoclonal Ig or resulting from abnormalities in the complement pathways (C3G) [16]; and diffuse proliferative GN is classified based on identification of underlying infection (infection-related GN), cryoglobulin (cryoglobulinemic GN) or autoimmune disease such as lupus (lupus nephritis). Noninflammatory patterns of injury such as focal segmental glomerulosclerosis (FSGS) are also now classified based on the underlying etiology: FSGS due to a presumed circulation permeability factor, FSGS associated with specific genetic mutations (genetic FSGS) and FSGS associated with specific conditions such as obesity, viral infections, drugs, etc. [17, 18].
Similarly, in this report, we propose that MN is not a specific disease but represents a pattern of injury resulting from specific disease entities related to the specific antigen/protein identified (Figure 1), as was suggested even before the discovery of the new specific antigens [19]. The membranous pattern is defined as thickened GBM with or without spikes or pinholes on LM, granular IgG with or without C3 on immunofluorescence (IF) and SE electron-dense deposits on EM. The clinical and pathologic characteristics of each newly discovered ‘antigens’ associated with MN are quite specific to the ‘antigen’ and these should be labeled as specific disease entities (Table 1). While PLA2R and THSD7A are well studied, in the following paragraphs, we highlight key unique features of each new ‘antigen’-associated MN and argue that each represents a distinct disease entity and should be labeled as such.
FIGURE 1:
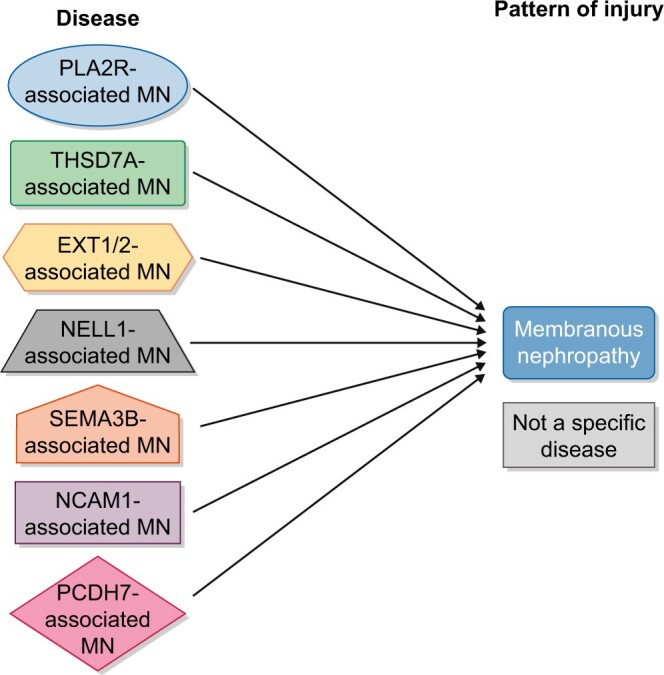
Schematic figure of MN. Different diseases are represented in different shapes such as PLA2R-associated MN, THSD7A-associated MN, NELL1-associated MN, EXT1/2-associated MN, SEMA3B-associated MN, NCAM1-associated MN and PCDH7-associated MN, all of which result in the membranous pattern of injury. The membranous pattern is defined as thickened GBM with or without spikes or pinholes on LM, granular IgG with or without C3 on IF and SE (±intramembranous) electron-dense deposits on EM.
Table 1.
The newly discovered protein/antigens-associated MN represents distinct diseases with regards to clinical and pathologic findings
| New protein/Ag-MN (approximate %) | Agea/sex | Disease/others | LM (proliferative GN) | IF | IgG subtype | EM | Circulating antibodies |
|---|---|---|---|---|---|---|---|
| PLA2R (50–60%) | 54/M > F (4:1) | Usually none | Negative | IgG, C3 | IgG4 | SE | + (nr) |
| THSD7A (1–2%) | 49/F > M (5:1) | Malignancy | Negative | IgG, C3 | IgG4 | SE | + (nr) |
| EXT1/EXT2 (10–20%) | 37/F > M (4:1) | Autoimmune disease | ± | Full house Ig, C3, C1q | IgG1 | SE, SU ± ME | ND |
| NELL1 (3–8%) | 63/M = F | Malignancy | Negative | IgG, C3 | IgG1 | Segmental SE | + (nr) |
| SEMA3B (1–3%) | 7 and 36b/M > F (6.4:3.6) | Geneticc | Negative | IgG, C3, TBM IgG | IgG1 | SE TBM deposits | + (r) |
| PCDH7 (2–5%) | 61/M > F (2.3:1) | Variable | Negative | IgG, negative/minimal C3, C1q | IgG1 and IgG4 | SE | + (r) |
| NCAM1 (2–3%) | 34/F > M (2.3:1) | Autoimmune disease | ± | Full house Ig (40%), C3, C1q | No specific subtype | SE, SU ± ME | + (nr) |
Average age.
Bimodal age presentation.
Genetic basis inferred as SEMA3B-MN was detected in siblings.
ND, not detected; r, reducing conditions; nr, nonreducing conditions.
EXT1/EXT2-ASSOCIATED MN
EXT1/EXT2-associated MN occurs at a younger age and is more common in females (4:1) [7]. Most importantly, EXT1/EXT2-associated nephropathy is associated with an underlying autoimmune disease such as systemic lupus erythematosus and mixed connective tissue disease. In rare cases with no known autoimmune disease, EXT1/EXT2-associated nephropathy may in fact be a marker for development of subsequent autoimmune disease. EXT1/EXT2-associated nephropathy does not show a temporal association with malignancy, infection or drugs.
The kidney biopsy findings in EXT1/EXT2-associated MN are unique in that a proliferative component may be present in a subset of EXT1/EXT2-positive lupus MN (LMN), IF microscopy often shows a full house pattern of staining with IgG, IgM, IgA, C1q and C3 with the IgG1 representing the dominant IgG subclass, and EM showing SE, mesangial (ME) and sometimes subendothelial (SU) electron-dense deposits. It should be pointed out EXT1/EXT2 staining occurs only along the GBM and that there is no ME EXT1/EXT2 staining in EXT1/EXT2-positive LMN even though ME deposits are also present.
Serum antibodies have not yet been detected in EXT1/EXT2-associated nephropathy.
Finally, a recent study has shown that EXT1/EXT2-positive LMN shows favorable biopsy findings and clinical outcomes compared with EXT1/EXT2-negative LMN [20].
NELL1-ASSOCIATED MN
NELL1-associated MN occurs in older patients (63.4 ± 10.4 years) with an almost equal male-to-female ratio [8]. In a subgroup of patients of NELL1-associated MN, a coexisting malignancy may be present. NELL1-associated MN is the second most common cause of MN pattern of injury after PLA2R-associated MN [21].
The kidney biopsy shows thickened GBM on LM, bright IgG and C3 staining along the GBM on IF microscopy with IgG1 being the dominant IgG subclass and SE electron-dense deposits on EM. Interestingly, the SE deposits in NELL1-associated MN can be segmental along the capillary walls [22].
Antibodies to NELL1 are detected in NELL1-associated MN; the antibodies are not detected under reducing conditions, suggesting that the NELL1 autoantibody recognizes conformation-dependent epitopes.
SEMA3B-ASSOCIATED MN
SEMA3B-associated MN is primarily present in pediatric patients (6.9 ± 6.8 years) [9]. It is also present in young adult patients aged 36.3 (standard deviation ±7.2) years. In almost 50% of cases, SEMA3B-associated MN is detected below the age of 2 years. SEMA3B-associated MN is a very rare cause of MN. SEMA3B-associated MN may have an underlying genetic basis as it occurs at a very young age and has been found in siblings and in a family (father and daughter) [9].
The kidney biopsy shows bright IgG and C3 along the GBM, with IgG1 being the dominant IgG subclass; and in the pediatric cases <2 years of age, there is also IgG staining along the tubular basement membrane (TBM). However, SEMA3B staining is present only along the GBM and not along the TBM.
Antibodies to SEMA3B are detected in SEMA3B-associated MN, and unlike NELL1-associated MN, the antibodies are detected under reducing conditions, suggesting that SEMA3B autoantibody may recognize a cryptic epitope that is unmasked by disruption of disulfide bonds, and that an event is required to disrupt the disulfide bonds and expose the epitope.
PCDH7-ASSOCIATED MN
PCDH7-associated MN is primarily present in older patients (61 ± 11.7 years). In one series, it appears to be the fourth most common after PLA2R-, EXT1/EXT2- and NELL1-associated MN [10]. Rarely, malignancy may be associated with PCDH7-associated MN.
The kidney biopsy shows thickened GBM, bright IgG staining along the GBM on IF microscopy and SE electron-dense deposits on EM. IgG subtypes show either predominantly IgG1 or IgG4. Importantly, C1q and C3 staining are minimal/absent.
Antibodies to PCDH7 are detected under nonreducing conditions in PCDH7-associated MN.
The proteinuria may be in the non-nephrotic range, and a subset of PCDH7-associated MN undergo remission with conservative management.
NCAM1-ASSOCIATED MN
NCAM1-associated MN occurs at a younger age (34 ± 12 years) and is more common in women (2.3:1) [11]. Ninety-one percent of NCAM1-associated MN have systemic lupus erythematosus. In addition, 40% patients have neuropsychiatric disease.
The kidney biopsy shows thickened GBM with proliferative features in 25% of the cases, bright IgG and C3 with full house Ig in 40% of cases on IF, and SE and ME electron-dense deposits in all cases and SU deposits in 21%.
Antibodies to NCAM1 are detected under nonreducing conditions.
SUMMARY
Taken together, the clinical presentation including age and sex, the kidney biopsy findings such as dual GBM staining for EXT1 and EXT2 (EXT1/EXT2-associated MN), segmental GBM staining (NELL1-associated MN) and TBM deposits (SEMA3B-associated MN), although TBM deposits are negative for SEMA3B staining or lack of complement staining (PCDH7-associated MN), detection of antibodies under nonreducing (NELL1- and SEMA3B-associated MN) and reducing conditions (PCDH7- and NCAM1-associated MN), and clinical outcomes including favorable outcomes in EXT1/EXT2-associated membranous lupus nephritis and remission with conservative treatment in PCDH7-associated MN, all point to distinct disease entities rather than a single disease entity of MN. Thus, we propose that each ‘antigen’ associated with MN pattern should be considered as specific disease and labeled as EXT1/EXT2-associated MN, NELL1-associated MN, SEMA3B-associated MN, PCDH7-associated MN and NCAM1-associated MN, recognizing that each disease results in an MN pattern of injury (Figure 1). Further studies are required to determine the pathogenesis, response to treatment and long-term outcomes of these new specific diseases. Lastly, any clinical trial of MN should take into account the specific antigens/protein-associated MN, even though some are rare, rather than lumping all cases together as a single MN disease entity.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Churg J, Ehrenreich T.. Membranous nephropathy. Perspect Nephrol Hypertens 1973; 1: 443–448 [PubMed] [Google Scholar]
- 2.Beck LH.PLA2R and THSD7A: disparate paths to the same disease? J Am Soc Nephrol 2017; 28: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couser WG.Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12: 983–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debiec H, Guigonis V, Mougenot B. et al. Antenatal membranous glomerulonephritis due to anti–neutral endopeptidase antibodies. N Engl J Med 2002; 346: 2053–2060 [DOI] [PubMed] [Google Scholar]
- 5.Beck LH, Bonegio RGB, Lambeau G. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomas NM, Beck LH, Meyer-Schwesinger C. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Madden BJ, Debiec H. et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 2019; 30: 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi S, Debiec H, Madden B. et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 2020; 97: 163–174 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Debiec H, Madden B. et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 2020; 98: 1253–1265 [DOI] [PubMed] [Google Scholar]
- 10.Sethi S, Madden BJ, Debiec H. et al. Protocadherin 7-associated membranous nephropathy. J Am Soc Nephrol 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caza TN, Hassen SI, Kuperman M. et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int 2020; doi: 10.1016/j.kint.2020.09.016 (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S.New ‘antigens’ in membranous nephropathy. J Am Soc Nephrol 2021; 32: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, Haas M, Markowitz GS. et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 2016; 27: 1278–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennette JC, Nachman PH.. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 2017; 12: 1680–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles Jennette J.Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003; 63: 1164–1177 [DOI] [PubMed] [Google Scholar]
- 16.Sethi S, Fervenza FC.. Membranoproliferative glomerulonephritis: a new look at an old entity. N Engl J Med 2012; 366: 1119–1131 [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Sethi S, Nath KA. et al. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 2018; 29: 759–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs-Cachá C, Vergara A, García-Carro C. et al. Challenges in primary focal segmental glomerulosclerosis diagnosis: from the diagnostic algorithm to novel biomarkers. Clin Kidney J 2021; 14: 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoxha E, von Haxthausen F, Wiech T. et al. Membranous nephropathy—one morphologic pattern with different diseases. Pflugers Arch 2017; 469: 989–996 [DOI] [PubMed] [Google Scholar]
- 20.Ravindran A, Casal Moura M, Fervenza FC. et al. In patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J Am Soc Nephrol 2021; 32: 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caza T, Hassen S, Dvanajscak Z. et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int 2021; 99: 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudose S, Santoriello D, Debiec H. et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int 2021; 99: 247–255 [DOI] [PubMed] [Google Scholar]


