Abstract
Background
Secondary hyperparathyroidism (SHPT) is a common and major complication in chronic kidney disease (CKD), reflecting the increase of parathyroid hormone (PTH) in response to reduced vitamin D signalling and hypocalcaemia. This meta-analysis evaluated the impact of nutritional vitamin D (NVD) (cholecalciferol or ergocalciferol) on SHPT-related biomarkers.
Methods
A systematic literature search was performed in PubMed to identify relevant randomized control trials to be included in the meta-analysis. Fixed- and random-effects models were used to pool study-level results. Effects were studied within NVD study arms and relative to control groups (placebo/no treatment); the former in order to identify the effect of actively altering biomarkers levels.
Results
Reductions in PTH from supplementation with NVD were small when observed within the NVD study arms (pooled reduction: 10.5 pg/mL) and larger when compared with placebo/no treatment (pooled reduction: 49.7 pg/mL). NVD supplementation increased levels of 25-hydroxyvitamin D [25(OH)D] in both analyses (increase within NVD study arm: 20.6 ng/mL, increase versus placebo/no treatment: 26.9 ng/mL). While small and statistically non-significant changes in phosphate and fibroblast growth factor 23 were observed, NVD supplementation caused calcium levels to increase when compared with placebo/no treatment (increase: 0.23 mg/dL).
Conclusions
Our results suggest that supplementation with NVD can be used to increase 25(OH)D to a certain extent, while the potential of NVD to actively reduce PTH in non-dialysis-CKD patients with SHPT is limited.
Keywords: meta-analysis, non-dialysis chronic kidney disease, PTH, secondary hyperparathyroidism, vitamin D supplementation
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a common and major complication in chronic kidney disease (CKD), and is frequent in both patients with non-dialysis CKD (ND-CKD) and patients requiring dialysis [1, 2]. SHPT is characterized by the increase of parathyroid hormone (PTH) in response to reduced vitamin D signalling and hypocalcaemia induced by phosphate retention and/or fibroblast growth factor 23 (FGF23) increase as a consequence of decreasing kidney function. Prolonged parathyroid gland stimulation leads to parathyroid hyperplasia with a progressive decrease in number and sensitivity of the vitamin D, calcium-sensing and FGF23 receptors, ultimately resulting in severe and uncontrolled SHPT. If left untreated, SHPT can cause bone and cardiovascular disease and result in increased rates of fractures, vascular calcification, morbidity and mortality in CKD patients [3–5]. SHPT has also been associated with a faster CKD progression [5].
The mainstays of early treatment of SHPT are the reduction of PTH by limiting phosphate load and an increase in vitamin D, while at the same time trying to avoid adverse changes in parameters such as calcium, phosphate and FGF23. Phosphate load and low levels of calcifediol [25-hydroxyvitamin D, hereafter 25(OH)D] and calcitriol [1,25-dihydroxyvitamin D, hereafter 1,25(OH)2D] are considered critical factors in the onset and progression of SHPT [1, 6] and the prevalence of vitamin D insufficiency or deficiency is higher among patients with kidney disease compared with the general population [7–9].
25(OH)D is hydroxylated to 1,25(OH)2D by the enzyme 1α-hydroxylase (CYP27B1) in several tissues in addition to the kidney. The 1,25(OH)2D synthesized in extrarenal tissues mainly performs autocrine or paracrine cell-specific roles. Furthermore, in contrast to that originating from the kidney, extrarenal production of 1,25(OH)2D does not significantly contribute to the circulating pool of 1,25(OH)2D [6, 8–10].
To address deficiency of vitamin D in the CKD population, existing clinical guidelines recommend supplementation with nutritional vitamin D (NVD), i.e. cholecalciferol or ergocalciferol, although clinical benefits from such supplementation have not been verified, especially in patients with ND-CKD for the treatment of e.g. SHPT [11, 12]. At the same time, routine use of active vitamin D and active vitamin D analogues is no longer recommended in earlier stages of CKD after having been shown to increase calcium, phosphate and FGF23, and increase the risk of hypercalcaemia in recent trials [11, 13, 14]. Instead, these agents should be reserved for severe and progressive SHPT in CKD Stages 4–5 [11].
Definition of an effective treatment strategy of SHPT in ND-CKD, starting from NVD, is further complicated by several factors: (i) there is no consensus on vitamin D status assessment and what constitutes adequate or toxic levels of vitamin D in ND-CKD patients; (ii) there is no agreement about target levels of PTH in ND-CKD; and (iii) the interrelationship between PTH and vitamin D is not fully understood [11, 12].
The pathways involved in the PTH-lowering effect of NVD supplementation are unclear. Parathyroid glands have been shown to express 1α-hydroxylase, so suggesting a possible autocrine mechanism where circulating 25(OH)D can be converted to 1,25(OH)2D within parathyroid cells which in turn binds locally to the vitamin D receptor [15]. However, it is unknown whether the level of 1α-hydroxylase activity in parathyroid glands is able to produce adequate amounts of 1,25(OH)2D to affect PTH secretion.
New evidence suggests that the increases in 25(OH)D levels able to induce meaningful reductions in PTH in ND-CKD are higher than previously thought. In a secondary analysis of two large randomized controlled trials (RCTs), Strugnell et al. [16] identified a 50.8 ng/mL threshold of 25(OH)D for significant PTH reductions while observing that gradual elevation of 25(OH)D to 92.5 ng/mL was not associated with significant adverse changes in safety parameters. In a large sampled cross-sectional study, Ennis et al. [17] found indications that optimal levels of 25(OH)D were in a similar range.
A recent study indicates that the potential of NVD supplementation to lower high PTH may be limited in CKD patients [18]. The authors found that PTH reductions in patients receiving supplementation with cholecalciferol were minor, but that these reductions appeared substantial when compared with the placebo group, in which PTH levels increased over the study period.
In this analysis, we re-assess the topic of NVD supplementation to target SHPT in ND-CKD by synthesizing results from the growing body of evidence from RCTs using meta-analytic methods. Specifically, the objective of this analysis was to investigate the effectiveness of NVD supplementation to actively lower PTH and raise vitamin D in ND-CKD patients with elevated PTH levels. To be able to distinguish between an actual decrease of PTH and relative lower levels we measured the effects of NVD supplementation on PTH and 25(OH)D in two ways: as changes before versus after NVD supplementation limited to the patients that received NVD (without comparison with a control group) and as changes in biomarker levels compared with control groups comprising placebo or untreated patients.
Secondary objectives included investigating the ability of NVD to raise levels of 25(OH)D above clinically meaningful thresholds and evaluating the effects of supplementation with NVD on the safety-related biomarkers calcium, phosphate and FGF23.
MATERIALS AND METHODS
Study selection and data extraction
To identify articles for inclusion in the meta-analysis, a systematic literature review was conducted in PubMed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [19]. A publication was included in the meta-analysis if it presented results from an RCT that evaluated one or both of the NVD supplements ergocalciferol and cholecalciferol, and had a study population of at least 20 adult (18+ years) patients with documented ND-CKD. All stages of the study selection and data extraction were performed independently by two reviewers.
The main results extracted from the included studies were changes in absolute values of the biomarkers PTH, 25(OH)D, calcium, phosphate and FGF23 from start to end of the study periods for the patient groups in the study. We contacted authors of publications where results were reported as median and interquartile ranges (IQRs) to obtain corresponding mean and standard deviations (SDs). Still, median results for PTH from two publications had to be used [20, 21]. The IQRs reported in these publications were converted to SDs by dividing the range of the IQRs by 1.35 [22].
Outcomes
The main treatment outcomes in this study were changes in absolute values of the biomarkers PTH and 25(OH)D.
Treatment outcomes were measured in two different ways. First, the outcomes were measured as the change in biomarker values from baseline to end of study within the study arm receiving NVD (‘difference within the NVD study arm’). When expressed this way, biomarker changes in control groups did not have an influence on the results, aiming to capture the potential of NVD to actively increase or decrease the biomarkers in patients receiving supplementation. Second, the outcomes were measured as the difference in biomarker values at baseline and end of the study in the study arms receiving NVD relative to the study arms receiving placebo treatment or no treatment (‘difference versus placebo/no treatment’). When defined this way, changes in biomarker levels in both the NVD study arms and the placebo/no treatment arms contributed to the measured effect.
The ability of NVD to raise levels of 25(OH)D above certain thresholds was assessed using the end-of-study 25(OH)D levels in the NVD study arms.
Values for PTH and FGF23 were transformed to picograms per millilitre (pg/mL), 25(OH)D to nanograms per millilitre (ng/mL), and calcium and phosphate to milligrams per decilitre (mg/dL).
Quality of evidence
The quality of the evidence for the outcomes on PTH and 25(OH)D was assessed to be of high, moderate, low or very low quality using the methodology of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group [23].
Statistical analysis
Fixed- and random-effects models were used to combine study-level results into overall, pooled effects. Inverse variance weighting was applied in the fixed-effects model and the weighting of study level results in the random-effects model was based on the DerSimonian and Laird method.
Sensitivity analyses were performed where studies with a high assessed risk of bias and publications reporting results other than mean measures were excluded. Meta-regressions using variables related to patient and study characteristics were performed to explore possible sources of heterogeneity in the results. All analyses were performed in Stata version 16.
RESULTS
Study selection and patient characteristics
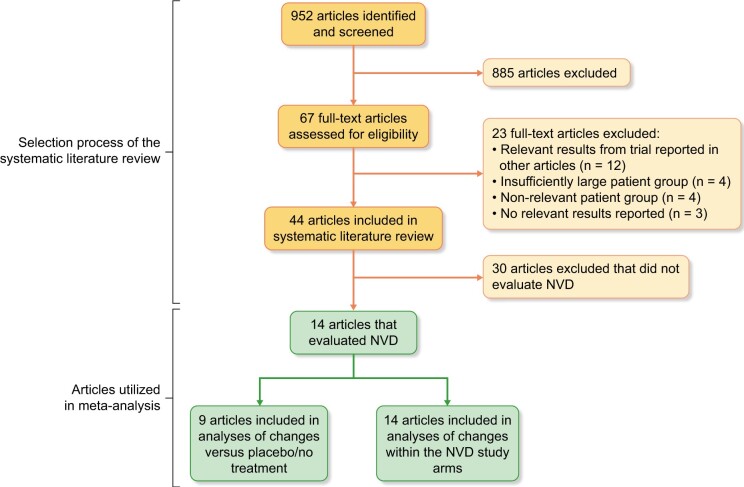
The initial search was conducted on 3 January 2019 and yielded a total of 952 hits. Fourteen of the identified studies fulfilled the inclusion criteria and evaluated at least one NVD, and were hence included in the meta-analysis. Figure 1 shows the stepwise identification of publications for the meta-analysis.
FIGURE 1:
Study selection.
Of the 14 studies, 7 included a placebo group [18, 21, 24–28] and two included a control group receiving no treatment [29, 30]. The five remaining studies [20, 31–34] included only study arms that received an active intervention, and no study arms receiving placebo-treatment or no treatment. Thus, they were excluded from the analyses where the outcome was compared with placebo/no treatment. One study [25] reported all results as geometric means. Only median values were available for PTH in two studies [20, 21]. Table 1 presents characteristics for the included studies and patient groups.
Table 1.
Selected studies and patient characteristics
| References | Study length | Risk of bias | Study arms | Dose | Number of patients | Age (years) | Percentage of female | Baseline eGFR (mL/min/ 1.73 m2) | Baseline PTH (pg/mL) | Baseline 25(OH)D (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
|
Panel A: Studies comparing NVD versus placebo or no treatment (used in analyses of change versus placebo/no treatment and in analyses of changes in NVD study arms) | ||||||||||
| Alvarez et al. [24] | 52 weeks | Moderate | Cholecalciferol | 50 000 IU/week first 12 weeks, 50 000 IU/ every other week rest of study (approximate average weekly dose of 30 800 IU) | 22 | 63.2 | 9.1 | 62.5 | 89.1 | 26.7 |
| Placebo | NA | 24 | 62.6 | 8.3 | 61.2 | 78.2 | 32.1 | |||
| Chandra et al. [25] | 12 weeks | High | Cholecalciferol | 50 000 IU/week | 17 | 62.2 | 60 | 33.3 | 288.9a | 17.3a |
| Placebo | NA | 17 | 59.5 | 60 | 29.8 | 290.5a | 18.6a | |||
| Dogan et al. [30] | 4 weeks | High | Cholecalciferol | 300 000 IU/month (only one treatment) (approximate average weekly dose of 75 000 IU) | 20 | 51 | 18 | 36.3 | 368 | 8.5f |
| No treatment | NA | 20 | 47 | 18 | 34.6 | 273 | 6.8f | |||
| Dreyer et al. [26] | 26 weeks | High | Ergocalciferol | 50 000 IU/week first month, 50 000 IU/month remaining 5 months (approximate average weekly dose of 18 300 IU) | 20 | 45.8 | 39.1 | 33 | 102.8 | NA |
| Placebo | NA | 18 | 48.8 | 26.3 | 38.7 | 118.9 | NA | |||
| Gravesen et al. [29] | 6 weeks | High | Ergocalciferol | 50 000 IU/week | 26 | NA | NA | 20.1 | 180.1 | 25 |
| No treatment | NA | 17 | NA | NA | 20.9 | 166.252 | 23.72 | |||
| Kumar et al. [27] | 16 weeks | Moderate | Cholecalciferol | 300 000 IU/month (only two treatments) (approximate average weekly dose of 75 000 IU) | 60 | 43.2 | 29 | 35.8 | 139b | 13.4 |
| Placebo | NA | 60 | 45.2 | 32 | 34.6 | 146b | 13.2 | |||
| Marckmann et al. [28]d | 8 weeks | Moderate | Cholecalciferol | 40 000 IU/week | 13 | 71b,c | 27b,c | 32b,c | 79.2b | 15.7b |
| Placebo | NA | 12 | 68b,c | 23b,c | 29b,c | 119.8b | 11.4b | |||
| Petchey et al. [21] | 26 weeks | Moderate | Cholecalciferol | 2000 IU/day (approximate average weekly dose of 14 000 IU) | 13 | 65 | 46 | 39 | 84.9b | 38 |
| Placebo | NA | 15 | 67 | 13 | 41 | 103.7b | 35.2 | |||
| Westerberg et al. [18] | 12 weeks | Low | Cholecalciferol | 8000 IU/day (approximate average weekly dose of 56 000 IU) | 48 | 62.6 | 44 | 32 | 102.8 | 23 |
| Placebo | NA | 49 | 64 | 20 | 29.5 | 123.5 | 22.7 | |||
| Summary Panel A: | Approximate average weekly dose 45 448 IU | 471 (sum) | 52.5 (average) | 32.9 (average) | 35.7 (average) | 158.7 (average) | 20.7 (average) | |||
|
| ||||||||||
|
Panel B: Studies investigating an NVD compared with another NVD or a non-relevant comparator (used only in analysis of changes in NVD study arms) | ||||||||||
| Kendrick et al. [20] | 26 weeks | Very low | Cholecalciferol | 4000 IU/day first month, 2000 IU/day remaining 5 months (approximate average weekly dose of 16 200 IU) | 64 | 58 | 30 | 33.5 | 99.5c | 23 |
| Calcitriole | 0.25 μg/day first month, 0.5 μg/day months 2–6 (if no episode of hypercalcaemia) | 64 | 59 | 36 | 32.8 | 93.8c | 21.7 | |||
| Kovesdy et al. (2012) [34] | 16 weeks | Moderate | Ergocalciferol | Dosed according to baseline 25(OH)D levels (as recommended by KDOQI) (average weekly dose not available) | 40 | 67.6 | 0 | 30.9 | 175 | 17.2 |
| Paricalcitole | 1 μg/day | 40 | 69.3 | 2 | 32.1 | 168 | 16.3 | |||
| Moe et al. [33] | 13 weeks | High | Cholecalciferol | 4000 IU/day first month, 2000 IU/day remaining months (approximate average weekly dose of 18 300 IU) | 22 | 62 | 54 | 29.6 | 109 | 14 |
| Doxercalciferole | 0.5 μg/day | 25 | 65 | 36 | 36.4 | 106 | 15.1 | |||
| Wetmore et al. [31] | 12 weeks | Moderate | Ergocalciferol | 50 000 IU/week | 22 | 59.4 | 47.6 | 37.5 | 149 | 20.5 |
| Cholecalciferol | 50 000 IU/week | 22 | 56.7 | 65 | 42.5 | 76.6 | 20.9 | |||
| Zhang et al. [32] | Mean follow-up 144 weeks | Moderate | Ergocalciferol | 50 000 IU/week first 3 months, maintained at 50 000 IU/month according to 25(OH)D levels (approximate average weekly dose of 50 000 IU) | 104 | 57.4 | 28.8 | 41.6 | 109.7 | 15.14 |
| Calcitriole | 0.25 μg/day (dose adjusted in response to changes in 25(OH)D, Ca, P and PTH) | 100 | 56.3 | 34 | 42.9 | 89.5 | 14.9 | |||
| Summary Panel B: | Approximate average weekly dose 33 615 IU | 503 (sum) |
61.1 (average) |
32.4 (average) |
36.0 (average) |
117.6 (average) |
17.9 (average) |
|||
| Summary all studies: | Approximate average weekly dose 41 807 IU | 974 (sum) |
55.8 (average) |
32.7 (average) | 35.8 (average) | 144.0 (average) | 19.6 (average) | |||
Numbers refer to geometric mean value.
Numbers refer to median values.
Numbers include measures for the full population of both dialysis and ND patients included in the study.
In the publication by Marckmann et al., only median value changes are reported, however, mean value changes for both PTH and 25(OH)D were obtained from the authors via e-mail, and as such, the results presented in the Results sections below refer to mean values and not median values.
The values from these comparator arms will not be used in any of the analyses performed in this meta-analysis.
In the publication by Dogan et al., values are reported as pg/mL of calcidiol. No change in the estimated values or conversion of units has been made for these values in any of the analyses performed on 25(OH)D.
NA, not applicable; KDOQI, Kidney Disease Outcomes Quality Initiative; Ca, calcium; P, phosphorus.
Quality of evidence
The outcome of changes in PTH within the NVD study arms was assessed to be of moderate quality, due to the large number of studies available for inclusion and moderate levels of heterogeneity in the study level results. The two outcomes of change in PTH versus placebo/no treatment and the outcome of change in 25(OH)D within the NVD study arms were assessed to be of low quality, given a moderate number of available studies and substantial amounts of heterogeneity in the study level results, respectively. Finally, the outcome of changes in 25(OH)D versus placebo/no treatment was evaluated to be of very low quality because of the moderate number of articles available for inclusion and the substantial levels of heterogeneity in study-level results. Funnel plots for the four outcomes are shown in Supplementary data, Figure S1 in the Supplementary Materials.
Results on PTH
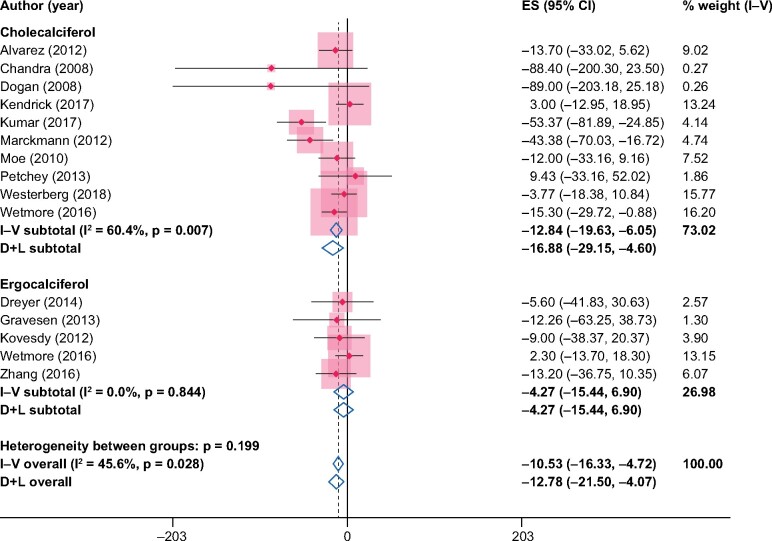
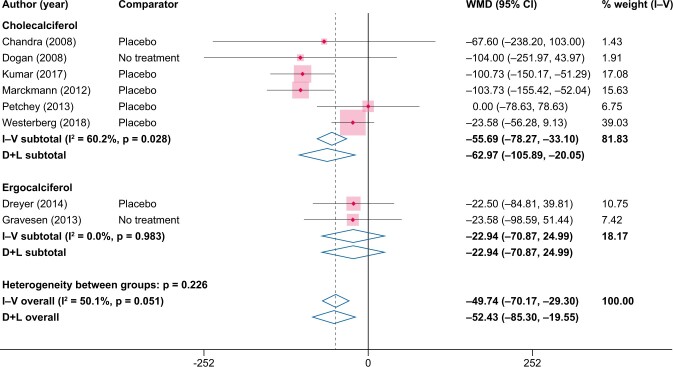
When estimated as the difference from baseline to end of study within the NVD study arm, the pooled difference in PTH in the NVD study arms was −10.5 pg/mL [95% confidence interval (CI) −16.3 to −4.7] from the fixed-effects model and −12.8 pg/mL (95% CI −21.5 to −4.1) from the random-effects model (Figure 2). When instead compared versus placebo/no treatment, the pooled difference in PTH was larger: −49.7 pg/mL (95% CI −70.2 to −29.3) from the fixed-effects model and −52.4 pg/mL (95% CI −85.3 to −19.6) from the random-effects model (Figure 3).
FIGURE 2:
Changes in PTH (pg/mL) from baseline to end of study in the NVD study arms. ES, effect size.
FIGURE 3:
Changes in PTH (pg/mL) from NVD supplementation versus placebo/no treatment. WMD, weighted mean difference.
Results on 25(OH)D
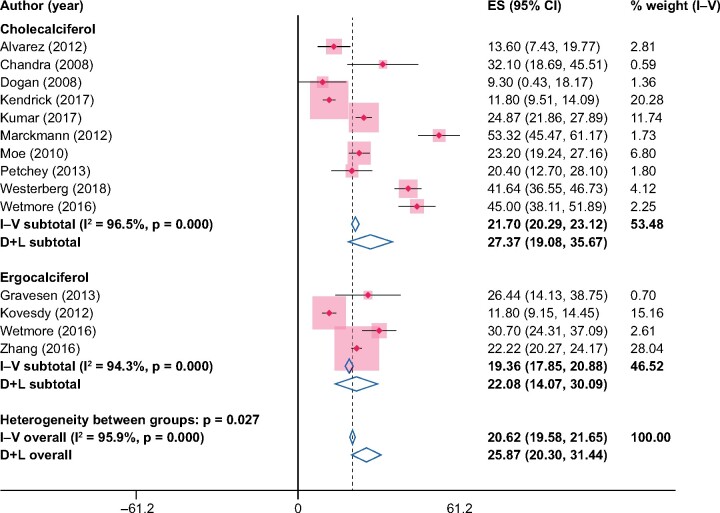
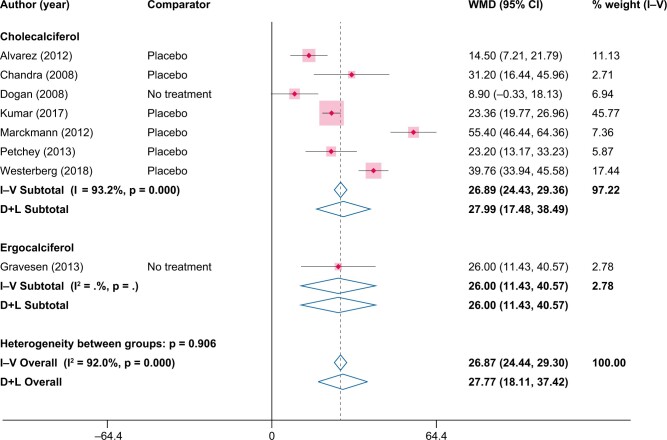
The pooled increase in 25(OH)D in within the NVD-study arms was 20.6 ng/mL (95% CI 19.6–21.7) from the fixed-effects model and 25.8 ng/mL (95% CI 20.3–31.4) from the random-effects model (Figure 4). The pooled increases in 25(OH)D were similar when compared versus placebo/no treatment, with a pooled increase of 26.8 ng/mL (95% CI 24.4–29.3) from the fixed-effects model and 27.8 ng/mL (95% CI 18.1–37.4) from the random-effects model (Figure 5). When compared with placebo/no treatment, the sizes of study level results on 25(OH)D varied considerably, ranging from 8.9 ng/mL [30] to 55.4 ng/mL [28]. At the end of the study periods, the average levels of 25(OH)D in the NVD study arms was >30 ng/mL in all but two studies [30, 34] and >50 ng/mL in five of the included studies [18, 21, 28, 29, 31] (Supplementary data, Table S1 in the Supplementary Materials).
FIGURE 4:
Changes in 25(OH)D (ng/mL) from baseline to end of study in the NVD study arms, ES, effect size.
FIGURE 5:
Changes in 25(OH)D (ng/mL) from NVD supplementation versus placebo/no treatment. WMD, weighted mean difference.
Sensitivity analyses and meta-regressions
Sensitivity analyses that excluded publications with a high assessed risk of bias and publications reporting non-mean results (median or geometric mean) showed similar results as for the main analyses (Supplementary data, Figures S2–S5). Meta-regression indicated that PTH reductions are larger in studies where baseline values of 25(OH)D are lower, with a 1 ng/mL increase in baseline 25(OH)D corresponding with an approximate 1.9 pg/mL lower reduction in PTH (Panel b of Supplementary data, Figure S6; P = 0.035). Meta-regressions using the other covariates mean age, percent of female participants, baseline values of eGFR, baseline values of PTH, assessed risk of bias and study length yielded no definite conclusion as to whether any of these characteristics explain the heterogeneity found in the study level results (Supplementary data, Figures S6 and S7).
Results on calcium, phosphate and FGF23
Supplementation with NVD significantly increased levels of calcium versus placebo/no treatment, with a pooled increase of 0.23 mg/dL (95% CI 0.12–0.34 mg/dL) from the fixed-effects model and 0.24 mg/dL (95% CI 0.08–0.40 mg/dL) from the random-effects model (Supplementary data, Figure S8). Versus placebo/no treatment, there were no statistically significant effects found on levels of phosphate or FGF23 (Supplementary data, Figures S9 and S10) from supplementation with NVD. Incidence rates of hypercalcaemia and hyperphosphataemia were rarely reported in the included publications and were low when reported.
DISCUSSION
We performed a meta-analysis on the effects of supplementation with NVD on several biomarkers in patients with ND-CKD, using evidence identified in a systematic review of RCTs. The results showed that supplementation with the NVD cholecalciferol and ergocalciferol caused only marginal PTH reductions in the NVD study arms. Differences in PTH were more pronounced when compared with changes in study arms receiving placebo treatment or no treatment, as PTH in the comparison groups often increased further. This indicates that NVD might be effective in preventing further increases in PTH but less effective in reducing PTH in patients with significantly increased levels.
Supplementation with cholecalciferol and ergocalciferol raised levels of 25(OH)D in all analyses, but large variations in the sizes of the study level effects make precise gauging of a true overall effect size difficult. Analyses of end-of-study values of 25(OH)D indicate that NVD can likely be used to raise 25(OH)D >30 ng/mL, while there is limited potential to raise 25(OH)D above the approximate threshold of 50 ng/mL, which might be necessary for clinically meaningful PTH responses in CKD patients as suggested by Strugnell et al. [16] and Ennis et al. [17]. Indeed, there are studies showing that increasing 25(OH)D levels above such thresholds can be achieved by providing calcifediol in a slow and gradual manner, which can be achieved with an extended-release formulation of calcifediol (ERC). ERC is indicated for the treatment of SHPT in CKD Stages 3 and 4 patients and vitamin D insufficiency or deficiency and has been shown to gradually raise serum 25(OH)D, resulting in progressive but physiologically controlled increases in serum levels of 1,25(OH)2D and sustained reductions in PTH levels without signs of any clinically relevant increases in serum phosphate and calcium [11, 16, 35].
Additional analyses showed statistically significant increases in calcium from supplementation with NVD versus placebo/no treatment. The observed effects on phosphate and FGF23 were minimal and incidences of hypercalcaemia and hyperphosphataemia were very low, corroborating the findings of limited toxicity from high levels of 25(OH)D in Strugnell et al. [16].
Previous meta-analyses on vitamin D supplementation in CKD were not planned to study solely ND-CKD patients and lacked sufficient statistical power from RCTs to draw any strong conclusions for the cohort of patients with ND-CKD. Kandula et al. [35] included six observational studies and two RCTs that reported results for patients with ND-CKD. The pooled results from the observational evidence in patients with ND-CKD show an increase of 19 ng/mL in 25(OH)D and a 26 pg/mL reduction in PTH from supplementation with NVD, while the pooled results from the two RCTs comprising ND-CKD patients are not reported separately.
In a more recent meta-analysis, Agarwal and Georgianos [36] identified four RCTs that evaluated vitamin D supplementation in ND-CKD. In an erratum to the original article, the authors present statistically significant pooled increases in 25(OH)D ranging from 12 to 21 ng/mL (depending on the model used) and statistically significant lowering of PTH when compared with placebo ranging from −62 to −58 pg/mL from supplementation with NVD. Nonetheless, the substantial heterogeneity in study-level effects and the low number of studies and patients included in the analysis lead the authors to conclude that recommendations to use NVD in ND-CKD are not justified based on their evidence.
While confirming the conclusions that vitamin D supplementation can increase levels of 25(OH)D compared with placebo or no treatment as presented in Kandula et al. [35] and Agarwal and Georgianos [36], the present meta-analysis provides evidence that levels of 25(OH)D tend to increase in patients receiving NVD supplementation, eliminating potential concerns that previously published results are driven by reductions in 25(OH)D in comparator groups.
Concerning the effects of NVD on levels of PTH, our findings indicate statistically significant reductions in PTH from NVD supplementation in all analyses. Nonetheless, our dual analyses of effects as compared with placebo/no treatment and of the effects in the NVD study arms show that only a small part of the difference versus placebo/no treatment can be attributed to PTH decreases in patients receiving NVD. The analyses of changes in PTH within the NVD study arms show a statistically significant but low reduction in PTH, which might be of limited benefit for patients that suffer from elevated levels of PTH or SHPT. The benefits from supplementation with NVD on PTH levels may therefore be interpreted as stemming largely from an avoidance of further elevations in PTH levels, rather than actively lowering PTH. Supplementation with NVD might consequently be of more use to patients that are early in the development of SHPT, while being limited in efficacy for patients with more advanced SHPT and elevated levels of PTH. In a recent study, Westerberg et al. [18] reached a similar conclusion after observing marginal PTH reductions from NVD supplementation and increases in PTH in placebo-treated patients. In their study, however, baseline values of PTH and share of patients in CKD Stage 4 were higher in the placebo arm than in the NVD arm (P-value between groups = 0.09 for both PTH and CKD stage distribution), indicating that patients in the placebo-arm had more progressed SHPT at baseline and were prone to additional PTH increases. Additionally, a meta-regression indicated that PTH reductions are larger in studies where baseline values of 25(OH)D are lower, seemingly reflecting a lack of impact from NVD on PTH in patients with less severe vitamin D insufficiency. The results from this meta-regression should be interpreted with some caution, however, as the correlation is driven to a large degree by two studies with a high risk of bias [25, 30] and two studies with a moderate risk of bias [27, 28].
The present analysis is not without limitations. At the study level, limitations stem from a combination of occasional lack of transparency in study designs, non-blinding of treatment allocation and non-standard reporting of effects, reducing the reliability of the pooled effects. For this reason, sensitivity analyses were performed using a subset of more comparable and higher-quality studies, which gave no indication that low-quality and potentially incomparable effect estimates distort the overall results.
Additionally, large variations in effect sizes at the study level caused moderate to high levels of heterogeneity in all outcomes, thereby making it difficult to draw any conclusions on what the true effect sizes may be. To investigate this heterogeneity, meta-regressions were performed using a broad set of covariates relating patients and study characteristics, but yielded no conclusive results on what underlying characteristics may cause the heterogeneous results. As such, uncertainty regarding the variation in effect sizes remains. Furthermore, investigation of any long-term effects is prohibited by the relatively short study durations (average approximate 6 months) in the included studies. Measures of uncertainty are also inherent in the biomarker outcomes analysed in this study, e.g. due to seasonal variability and variation over test assays. In particular, PTH is known to have a wide variance within patients over relatively short time spans. Therefore, repeated measurements at the end-of-studies might yield a more reliable effect measure. To our knowledge, however, the effects of NVD on the biomarkers are evaluated by a single measurement in the included studies, which could contribute to the heterogeneity observed in the study-level results.
This meta-analysis presents, to the best of our knowledge, the most comprehensive evidence to date regarding the effects from supplementation with NVD on the biomarkers PTH and 25(OH)D in ND-CKD patients based on the randomized clinical evidence. Our novel approach of separating the effects compared with study arms receiving placebo or no treatment and the effects within the NVD study arms shines a new light on the underlying mechanisms of vitamin D supplementation in ND-CKD. Our results suggest that supplementation with NVD in patients with ND-CKD is probably useful in raising low levels of 25(OH)D. However, the increase in 25(OH)D levels is often not sufficiently large to induce PTH-lowering effects and to meaningfully reduce levels of PTH in patients with SHPT.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
J.G. and R.L. are employees of Quantify Research, P.C. and E.K. are employees of Vifor Pharma. J.B. reports personal fees from several pharmaceutical companies. G.C. has nothing to disclose. Medical writing assistance was utilized in this manuscript and was funded by Vifor Fresenius Medical Care Renal Pharma.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
REFERENCES
- 1.Cunningham J, Locatelli F, Rodriguez M.. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011; 6: 913–921 [DOI] [PubMed] [Google Scholar]
- 2.Scialla JJ, Astor BC, Isakova T. et al. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 2013; 24: 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigwekar SU, Tamez H, Thadhani RI.. Vitamin D and chronic kidney disease-mineral bone disease (CKD-MBD). Bonekey Rep 2014; 3: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng S, Kuang Z, Peissig PL. et al. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int 2019; 30: 2019–2025 [DOI] [PubMed] [Google Scholar]
- 5.Schumock GT, Andress DL, Marx SE. et al. Association of secondary hyperparathyroidism with CKD progression, health care costs and survival in diabetic predialysis CKD patients. Nephron Clin Pract 2009; 113: c54–c61 [DOI] [PubMed] [Google Scholar]
- 6.Lishmanov A, Dorairajan S, Pak Y. et al. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int Urol Nephrol 2012; 44: 541–547 [DOI] [PubMed] [Google Scholar]
- 7.Doorenbos CR, van den Born J, Navis G. et al. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol 2009; 5: 691–700 [DOI] [PubMed] [Google Scholar]
- 8.Holick MF.Vitamin D deficiency. N Engl J Med 2007; 357: 266–281 [DOI] [PubMed] [Google Scholar]
- 9.Jean G, Souberbielle JC, Chazot C.. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017; 9: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dusso AS, Brown AJ, Slatopolsky E., . Vitamin D. Am J Physiol Renal Physiol 2005; 289: F8–F28 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO). Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; 113: S1–S130 [DOI] [PubMed] [Google Scholar]
- 13.Thadhani R, Appelbaum E, Pritchett Y. et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307: 674–684 [DOI] [PubMed] [Google Scholar]
- 14.Wang AYM, Fang F, Chan J. et al. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. J Am Soc Nephrol 2014; 25: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segersten U, Correa P, Hewison M. et al. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 2002; 87: 2967–2972 [DOI] [PubMed] [Google Scholar]
- 16.Strugnell SA, Sprague SM, Ashfaq A. et al. Rationale for raising current clinical practice guideline target for serum 25-hydroxyvitamin D in chronic kidney disease. Am J Nephrol 2019; 49: 284–293 [DOI] [PubMed] [Google Scholar]
- 17.Ennis JL, Worcester EM, Coe FL. et al. Current recommended 25-hydroxyvitamin D targets for chronic kidney disease management may be too low. J Nephrol 2016; 29: 63–70 [DOI] [PubMed] [Google Scholar]
- 18.Westerberg PA, Sterner G, Ljunggren Ö. et al. High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD Stages 3–4: results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 2018; 33: 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M. et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendrick J, Andrews E, You Z. et al. Cholecalciferol, calcitriol, and vascular function in CKD: a randomized, bouble-blind trial. Clin J Am Soc Nephrol 2017; 12: 1438–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petchey WG, Hickman IJ, Prins JB. et al. Vitamin D does not improve the metabolic health of patients with chronic kidney disease stage 3-4: a randomized controlled trial. Nephrology (Carlton )2013; 18: 26–35 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S.. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley, 2011 [Google Scholar]
- 23.Schünemann H, Guyatt G, Oxman A (eds); GRADE Working Group. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013) .2013; https://gradepro.org/cite/ [Google Scholar]
- 24.Alvarez JA, Law J, Coakley KE. et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2012; 96: 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra P, Binongo JNG, Ziegler TR. et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract 2008; 14: 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreyer G, Tucker AT, Harwood SM. et al. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: an exploratory, double blind, randomised controlled trial. PLoS One 2014; 9: e99461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar V, Yadav AK, Lal A. et al. A randomized trial of vitamin D supplementation on vascular function in CKD. J Am Soc Nephrol 2017; 28: 3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marckmann P, Agerskov H, Thineshkumar S. et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 2012; 27: 3523–3531 [DOI] [PubMed] [Google Scholar]
- 29.Gravesen E, Hofman-Bang J, Lewin E. et al. Ergocalciferol treatment and aspects of mineral homeostasis in patients with chronic kidney disease stage 4–5. Scand J Clin Lab Invest 2013; 73: 107–116 [DOI] [PubMed] [Google Scholar]
- 30.Dogan E, Erkoc R, Sayarlioglu H. et al. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail 2008; 30: 407–410 [DOI] [PubMed] [Google Scholar]
- 31.Wetmore JB, Kimber C, Mahnken JD. et al. Cholecalciferol v. ergocalciferol for 25-hydroxyvitamin D (25(OH)D) repletion in chronic kidney disease: a randomised clinical trial. Br J Nutr 2016; 116: 2074–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Li H, Yin D. et al. Ergocalciferol versus calcitriol for controlling chronic kidney disease mineral bone disorder in stage 3 to 5 CKD: a randomized controlled trial. Eur J Pharmacol 2016; 789: 127–133 [DOI] [PubMed] [Google Scholar]
- 33.Moe SM, Saifullah A, LaClair RE. et al. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovesdy CP, Lu JL, Malakauskas SM. et al. Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis 2012; 59: 58–66 [DOI] [PubMed] [Google Scholar]
- 35.Kandula P, Dobre M, Schold JD. et al. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 2011; 6: 50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal R, Georgianos PI.. Con: Nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 2016; 31: 706–713 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.