Abstract
Portal vein thrombosis (PVT) is a common complication in liver cirrhosis, especially in advanced cirrhosis. It may be related to a higher risk of liver-related events and liver function deterioration. Imaging examinations can not only provide an accurate diagnosis of PVT, such as the extent of thrombus involvement and the degree of lumen occupied, but also identify the nature of thrombus (i.e., benign/malignant and acute/chronic). Evolution of PVT, mainly including development, recanalization, progression, stability, and recurrence, could also be assessed based on the imaging examinations. This article briefly reviews the pathophysiology, diagnosis, classification, and evolution of PVT with an emphasis on their computed tomography imaging features.
INTRODUCTION
Portal vein thrombosis (PVT) is defined as a thrombus occupying the portal vein trunk and intrahepatic portal vein branches, sometimes with extension into the mesenteric vein or splenic vein (1). Owing to the progress of imaging techniques and medical awareness, PVT has been increasingly diagnosed. Liver cirrhosis is the most common cause of nonmalignant PVT (2). In turn, PVT is also a frequent complication of liver cirrhosis with a prevalence ranging from 1% to 26% (3,4), which increases with the severity of liver disease (5). The prevalence of PVT in compensated cirrhosis, decompensated cirrhosis, and liver transplantation candidates is 1%–5%, 10%–25%, and 8%–25%, respectively (5). PVT is associated with early mortality and graft failure after liver transplantation (6,7), although its effects on the outcomes of cirrhotic patients who are neither at the liver transplantation waiting list nor have undergone liver transplantations have not been fully elucidated (8,9). On the other hand, the influence of the dynamic change of PVT on the prognosis of patients with cirrhosis needs to be further elucidated. But, it seems that patients with progressed PVT have worse outcomes than those with improved or stable PVT (10,11). Early identification and assessment of PVT evolution potentially contributes to tailor treatment strategies, improving the prognosis and avoiding the potential risks related to invasive therapy. The current article aims to briefly review the pathophysiology, diagnosis, classification, and evolution of PVT, with an emphasis on imaging features. Management of PVT in liver cirrhosis, mainly including anticoagulation, thrombolysis, and transjugular intrahepatic portosystemic shunt, has been widely discussed by recent articles and guidelines (1,2,12) and is beyond the scope of this article.
PATHOPHYSIOLOGY OF PVT IN CIRRHOSIS
Virchow's triad—mainly including hypercoagulability, portal flow stasis, and vascular endothelial injury—is the mainstay explanation for the formation of PVT in patients with liver cirrhosis (4,13). Hypercoagulability is frequently observed in liver cirrhosis (14,15), which can manifest as decreased protein C (16), plasma metalloprotease ADAMTS 13 (a disintegrin and metalloprotease with thrombospondin type 1 motif 13) (17), and plasminogen (18), as well as increased factor VIII (19), von Willebrand factor (20), and plasminogen activator inhibitor (18). By comparison, portal flow stasis makes a greater contribution on the occurrence of PVT in liver cirrhosis (21). A reduced portal flow velocity of less than 15 cm/s increases the risk of PVT by 6- to 24-fold in patients with liver cirrhosis (22–24), but such an association is not confirmed by a prospective study (25). Factors causing direct or indirect damage to the portal vascular endothelium, such as intra-abdominal trauma or surgery (26,27), endoscopic variceal treatment (28), inflammation (29), or endotoxemia (30), can contribute to the development of PVT in liver cirrhosis.
DIAGNOSIS OF PVT IN CIRRHOSIS
Ultrasound is the first-line screening approach for the suspicion of PVT, whereas contrast-enhanced computed tomography (CT) images are highly accurate and reliable for the diagnosis of PVT, especially for the assessment of thrombosis extension (31,32). Partial PVT often manifests as a filling defect within the portal venous system lumen and complete PVT as an absence of contrast agents within the lumen. Once acute thrombus forms, it is likely that the diameter of the obstructed vessel will be enlarged (31), and acute/fresh thrombus sometimes manifests as hyperdense on non–contrast-enhanced CT images (33). If a chronic thrombus persists, the obstructed vessel would become obliterated and evolve into a fibrotic cord. Concomitantly, multiple small and tortuous vessels would develop around the obstructed intrahepatic portal branches, portal trunk, and/or superior mesenteric vein (SMV), a radiological finding that is referred to as a cavernous transformation of the portal vein (CTPV).
In the general population, the capture of portal venous phase images needs a delay of 60–70 seconds after the injection of contrast agents, but a greater delay of 80 seconds is needed in cirrhotic patients because of their decreased portal flow velocity (34). Failure in acquiring optimal contrast-enhanced CT images at the portal venous phase produces poor-quality images, in which contrast agents are insufficiently or hardly filled within the portal venous system lumen, leading to a false-positive radiological finding of partial or complete PVT (Figure 1). In addition, as the bile duct runs in parallel with the portal vein, a mildly dilated bile duct can be characterized as a low density near the portal vein on contrast-enhanced CT images, sometimes being mistaken as a thrombus occupying the portal vein lumen (Figure 2). For these reasons, the accuracy of the PVT diagnosis depends on the awareness about diagnostic pitfalls, which can be avoided by reviewing the images on multiple cross-sectional layers.
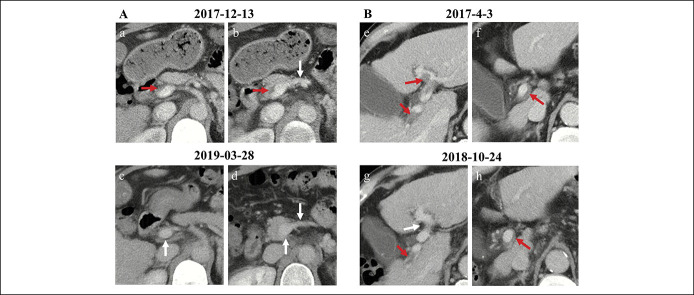
Figure 1.
Diagnostic pitfalls of PVT caused by poor-quality CT images. Contrast-enhanced axial CT scans at the portal venous phase showed a mural thrombus occupying the confluence of SMV and splenic vein (red arrow, a) and a complete thrombus occupying the SMV (red arrow, b). Contrast-enhanced axial CT scans at the equilibrium phase showed patent confluence of SMV and splenic vein (white arrow, c) and SMV (white arrow, d). Therefore, mural thrombus shown at portal venous phase may be due to insufficient filling of contrast agent in the confluence of SMV and splenic vein, and complete thrombus may be due to no filling of contrast agent in the SMV. CT, computed tomography; PVT, portal vein thrombosis; SMV, superior mesenteric vein.
Figure 2.
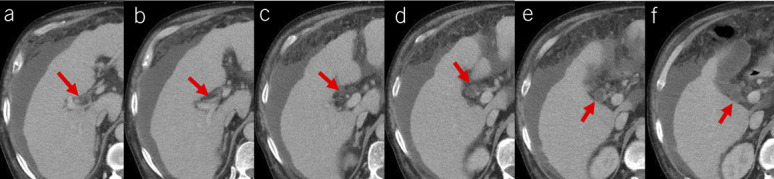
Diagnostic pitfall of PVT caused by a slightly dilated bile duct. Contrast-enhanced axial CT scans showed a thrombus-like hypodense occupying the right portal vein branch near the hepatic hilum (red arrow, a). After continuously reviewing multiple CT layers, such a thrombus-like hypodensity should be a slightly dilated bile duct (red arrows, b–f). CT, computed tomography; PVT, portal vein thrombosis.
Malignant invasion into the portal vein lumen is a common condition in patients with a confirmed diagnosis of hepatocellular carcinoma, which sometimes mimics PVT. Unlike benign PVT, which has an 1-year survival rate of 81%–96% after liver transplantation (35–37), malignant portal vein invasion has a median survival time of only 2.7 months (38) and is often considered a contraindication to liver transplantation (39). Therefore, how to differentiate between benign thrombus and malignant invasion is very important. Because malignant invasion is composed by tumor tissue, often rich in arterial blood supply, a punctate or linear enhancement in the “thrombus” can be seen at the arterial phase of contrast-enhanced CT scans (Figure 3). Besides, venous expansion, neovascularity, adjacence to hepatocellular carcinoma lesions, and disruption of vein walls are other contrast-enhanced CT features of malignant invasion (40–46). A combination of these features with an α-fetoprotein concentration of >1,000 ng/dL can diagnose malignant invasion with a sensitivity of 100% and a specificity of 93.6% (45). Contrast-enhanced ultrasonography (47) and magnetic resonance imaging (48) are alternative approaches for identifying tumoral invasion into portal vein. Certainly, pathological evaluation is still the gold standard criterion for distinguishing benign thrombus from malignant invasion.
Figure 3.
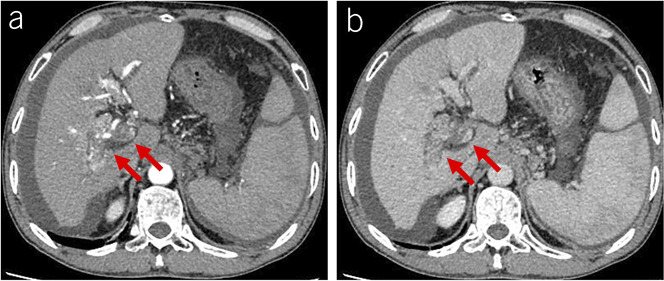
Malignant invasion into the portal vein in a patient with liver cirrhosis and hepatocellular carcinoma. (a) Contrast-enhanced CT scans at the arterial phase showed thread-like enhancement within the thrombus, venous expansion, and disruption of vein walls (red arrows). (b) Contrast-enhanced CT scans at the portal venous phase showed venous expansion and disruption of vein walls (red arrows). CT, computed tomography.
CLASSIFICATION OF PVT IN CIRRHOSIS
Identification of characteristics of PVT is of great significance to determine the necessity of anticoagulation therapy and feasibility of liver transplantation. Until now, at least 11 classifications have been developed to assess the degree, extent, and/or duration of PVT in patients with liver cirrhosis (46,49–59). Yerdel's classification (53) is the most widely used to select the type of liver transplantation procedure, according to the degree of thrombotic filling of the portal lumen and the involvement of SMV. However, this classification is limited to the evaluation of inherent portal venous system vessels, rather than that of spontaneous or surgical portosystemic shunts and large collaterals secondary to portal hypertension that can also be used for portal reconstruction during liver transplantation. Accordingly, Bhangui et al. (59) proposed a novel classification, which considers the presence of splenorenal shunt, large gastric vein, pericholedochal varix, and mesocaval shunt for guiding renoportal anastomosis, gastric vein-portal anastomosis, varix-portal anastomosis, and cavoportal anastomosis, respectively.
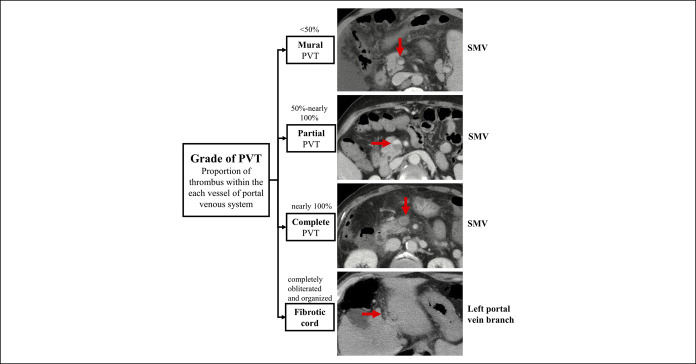
Except for evaluating the technical feasibility and outcomes of liver transplantation, the classifications of PVT should be worthwhile for assessing the need of antithrombotic treatment in non–liver transplantation candidates with liver cirrhosis. They should also consider the need of preventive or therapeutic strategies for portal hypertension–related complications. For this reason, the classification proposed by Sarin et al. (58) is more comprehensive in terms of the degree, extension, and duration of PVT. However, it seems a bit complicated. More recently, the Chinese consensus has proposed a simplified classification of PVT in liver cirrhosis based on the severity of thrombosis within each vessel (46) (Figure 4), which is potentially helpful to standardize the evolution of PVT and to evaluate the efficacy of anticoagulation therapy in a unanimous manner. Of course, the clinical applicability of any classification should be further confirmed.
Figure 4.
Classification of grade of PVT in liver cirrhosis according to the Chinese consensus. The red arrows indicate the thrombus. PVT, portal vein thrombosis; SMV, superior mesenteric vein.
The stage of PVT is traditionally classified as acute and chronic. Acute PVT is often considered when acute abdominal pain related to intestinal ischemia develops for a short duration of less than 60 days (56,58), which is often disproportionate to abdominal tenderness on physical examinations (60). Chronic PVT is defined as the presence of CTPV on images and/or abdominal symptoms for a duration of more than 60 days. However, it should be noted that the symptom, duration, and CTPV are not the independent criteria for staging PVT. Therefore, such a definition has been questioned (56,61). First, acute symptoms related to intestinal ischemia are noticeable only if the thrombus extends to the SMV (62). It is extremely rare in liver cirrhosis in clinical practice. Second, given that PVT is incidentally diagnosed in most patients with liver cirrhosis, it is often difficult to determine the onset of PVT. Third, CTPV rarely develops in the settings of partial PVT, despite its duration being longer than 3 months (56). By comparison, cavernous collateral vessels can develop as early as less than 6 days in the settings of occlusive PVT (63). Considering the limitations of the currently available staging system, the Chinese consensus has updated the stage of PVT as acute symptomatic and non-acute symptomatic to stratify the candidates who should undergo antithrombotic therapy and wait-and-see strategy, respectively (46). After a comprehensive evaluation of disease severity and adequate prevention of gastrointestinal bleeding, antithrombotic therapy should be immediately given in cirrhotic patients with acute symptomatic PVT, and surgeons should be timely consulted in those with a suspicion of intestinal ischemia. Decision of antithrombotic treatment is closely dependent on the grade and extent of PVT in cirrhotic patients with non-acute symptomatic PVT (46).
EVOLUTION OF PVT IN CIRRHOSIS
The evolution of PVT in cirrhosis mainly includes development, complete or partial recanalization, progression, stability, and recurrence (46).
Development of PVT
Development of PVT refers to the formation of de novo thrombus into the portal venous system in the absence of previous PVT (Figure 5). The cumulative 1-year incidence of PVT in cirrhosis is heterogeneous among studies, ranging from 4% to 18% (22,23,64,65). Most of de novo PVT are partial (64,66). The most common site for de novo PVT is the portal vein, followed by the SMV and splenic vein (23). A decreased portal vein flow velocity has been recognized as the most important predictor for de novo PVT in liver cirrhosis (64–66). Presence of large-size esophageal varices (66) and high-flow collateral varices (64,67) can produce the portal vein stealing effect, thus reducing the portal flow velocity and precipitating de novo PVT. In addition, the use of nonselective beta-blockers (NSBBs), which can significantly decrease heart rate and cardiac output and reduce portal blood flow, may be associated with an increased risk of PVT (25,68). However, it should be noted that such a potential harmful effect of NSBBs cannot counteract its benefits in the prevention of first variceal bleeding and variceal rebleeding which have been well confirmed by high-quality studies and recommended by mainstream practice guidelines and consensus (57,69).
Figure 5.
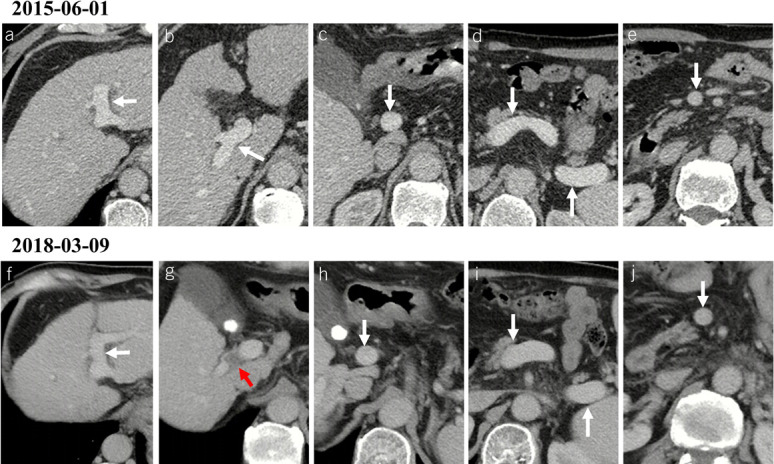
Development of PVT in a male patient with alcoholic liver cirrhosis. (a–e) Contrast-enhanced axial CT scans on June 1, 2015, showed patent portal venous system vessels, including left portal vein branch (a), right portal vein branch (b), portal vein trunk (c), confluence of SMV and splenic vein (d), splenic vein (d), and SMV (e). The score of each vessel was 0, 0, 0, 0, 0, and 0, respectively. (f–j) Contrast-enhanced axial CT scans on March 9, 2018, showed that de novo thrombus occupied the right portal vein branch (red arrow), while the other portal venous system vessels were still patent. The score of left portal vein branch, right portal vein branch, portal vein trunk, confluence of SMV and splenic vein, splenic vein, and SMV was 0, 2, 0, 0, 0, and 0, respectively. The total PVT score at baseline and during follow-up was 0 and 2, respectively, suggesting the development of PVT. Notes: The red arrows indicate the thrombus, and the white arrows indicate patent vessels. CT, computed tomography; PVT, portal vein thrombosis; SMV, superior mesenteric vein.
Recanalization of PVT
Recanalization of PVT is classified as complete or partial. Complete recanalization is defined as complete resolution of a previous thrombus into the lumen of the portal venous system (Figure 6). The definition of partial recanalization is inconsistent among the previous studies (11,70–76). Partial recanalization often refers to more than 50% reduction of previous thrombus without thrombus extension (70,71,74–76) (Figure 6).
Figure 6.
Recanalization of PVT in liver cirrhosis. (A) Complete recanalization of PVT in a male patient with hepatitis C virus–related cirrhosis. Contrast-enhanced axial CT scans on December 13, 2017, showed a thrombus occupying the portal vein trunk (a) and confluence of SMV and splenic vein (b). Contrast-enhanced axial CT scans on March 28, 2019, showed patent portal venous system vessels (c, d). (B) Partial recanalization of PVT in a male patient with alcoholic liver cirrhosis. Contrast-enhanced axial CT scans on April 3, 2017, showed a thrombus occupying the left portal vein branch (e), right portal vein branch (e), and portal vein trunk (f). Contrast-enhanced axial CT scans on October 24, 2018, showed that the thrombus within the left portal vein branch disappeared, but the previous thrombus remained within the right portal vein branch (g) and portal vein trunk (h). Notes: The red arrows indicate the thrombus, and the white arrows indicate patent vessels. CT, computed tomography; PVT, portal vein thrombosis; SMV, superior mesenteric vein.
Among patients with liver cirrhosis, spontaneous recanalization of PVT can be observed in the absence of antithrombotic treatment, which has been recognized as “transient PVT” (77). Its incidence ranges from 0% to 57% (3,7,11,64,70,75,76,78–82). A smaller diameter and flow volume of maximum portal collateral vessels may be associated with spontaneous recanalization of PVT (64). But, there may not be any significant influence of PVT (duration, degree, and location) and patient (severity of liver cirrhosis and portal hypertension) characteristics on spontaneous recanalization of PVT (11).
Recanalization of PVT in liver cirrhosis can be further improved by anticoagulation therapy, with an overall recanalization rate of 16.7%–80% and a complete recanalization rate of 23%–72% (3,70,76,78–84). Hepatic reserve, nature of PVT, and timing of anticoagulation therapy are related to the probability of recanalization of PVT. In details, Child-Pugh class B/C, high model for end-stage liver disease score (73,83), and presence of portal hypertension (78) are associated with decreased portal vein recanalization; more extensive PVT (85), complete thrombus (86), and thrombus age of more than 6 months (86) negatively correlate with portal vein recanalization; and early initiation of anticoagulation therapy can increase portal vein recanalization (70,78,87). In addition, recent evidence suggests that anticoagulation should improve survival (75,83,84) and hepatic function (88) in cirrhotic patients with PVT. However, whether such benefits are due to the use of anticoagulation itself or portal vein recanalization achieved by anticoagulation needs to be further explored.
Progression of PVT
Progression of PVT refers to an increase in the degree of a pre-existing thrombus in the portal venous system and/or an extension of a pre-existing thrombus into the previously patent vessels (Figure 7).
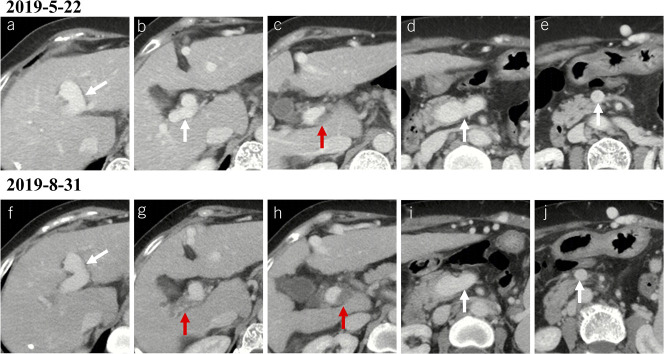
Figure 7.
Progression of PVT in a female patient with liver cirrhosis. (a–e) Contrast-enhanced axial CT scans on May 22, 2019, showed a thrombus occupying the portal vein trunk (c). (f–j) Contrast-enhanced axial CT scans on August 31, 2019, showed that the previous thrombus within the portal vein trunk had enlarged (h) with an extension to the right portal vein branch (g). Notes: The red arrows indicate the thrombus, and the white arrows indicate patent vessels. CT, computed tomography; PVT, portal vein thrombosis.
Progression is more common than spontaneous recanalization in cirrhotic patients with untreated PVT. Its incidence is approximately 0%–71% (3,11,66,70,78–81). Cirrhotic patients treated with NSBBs may have a higher risk of PVT progression than those without NSBBs (89). However, it should be noted that the evidence is insufficient from only 1 retrospective cohort study of 43 cirrhotic patients with a small number of progressing PVT events. On the other hand, cirrhotic patients who have received anticoagulation therapy have a 2- to 11-fold reduction in the risk of PVT progression (3,78,79).
Recurrence of PVT
Recurrence of PVT is defined as new thrombus development into the portal venous system after complete recanalization of a previous thrombus (Figure 8).
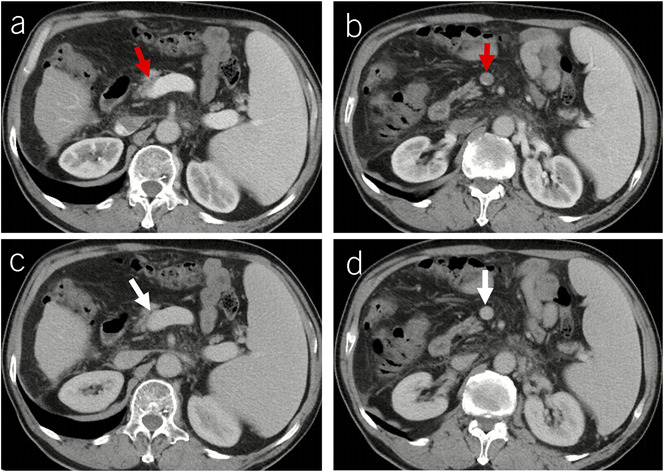
Figure 8.
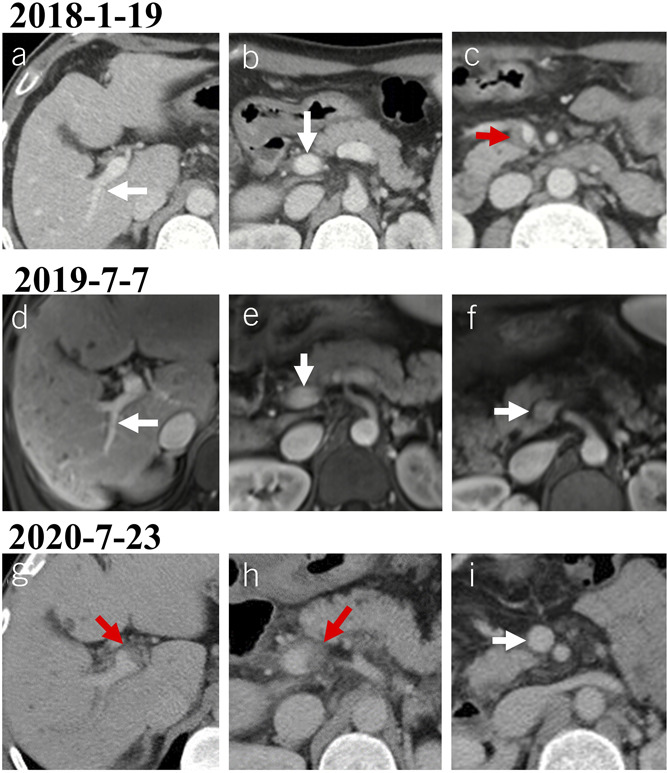
Recurrence of PVT after recanalization in a male patient with alcoholic liver cirrhosis. (a–c) Contrast-enhanced axial CT scans on January 19, 2018, showed a thrombus occupying the SMV (c). (d–f) Contrast-enhanced axial MRI scans on July 7, 2019, showed patent portal venous system vessels. (g–i) Contrast-enhanced axial CT scans on July 23, 2020, showed a new thrombus occupying the right portal vein branch (g) and portal vein trunk (h). Notes: The red arrows indicate the thrombus, and the white arrows indicate patent vessels. CT, computed tomography; MRI, magnetic resonance imaging; PVT, portal vein thrombosis; SMV, superior mesenteric vein.
In cirrhotic patients who develop spontaneous recanalization of PVT, the incidence of PVT recurrence is 4.2%–45% (64,66,80). Therefore, regular imaging examination is required to monitor the patency of the portal venous system lumen. Until now, no risk factor associated with recurrence of PVT has been identified in cases of spontaneous recanalization.
In cirrhotic patients who achieve portal vein recanalization after anticoagulant therapy, the incidence of PVT recurrence after termination of anticoagulation is 3.7%–53% (74,75,82,84,87,90–92). Elder thrombus, extensive thrombus, thrombogenic gene polymorphism, and use of warfarin correlate with PVT recurrence in cirrhotic patients receiving rivaroxaban or warfarin (74).
PVT score for evaluation of PVT evolution
Dynamic change of thrombus severity is often heterogeneous among different portal venous system vessels. For example, in the same patient, the degree of thrombus may be reduced while its extension is aggravated; or the degree of thrombus is decreased in some portal venous system vessels, but increased in others. Accordingly, we have attempted to holistically quantify PVT evolution by “PVT score” (89,93), which refers to the sum of the score calculated based on the proportion of thrombus occupying each portal venous system vessel, including left portal vein branch, right portal vein branch, portal vein trunk, confluence of SMV and splenic vein, splenic vein, and SMV. In details, less than 50% occlusion (mural thrombus), 50%–80% occlusion (partial thrombus), more than 80% occlusion (complete thrombus), and fibrotic cord are counted as 1, 2, 3, and 4 points, respectively (Figure 4).
CONCLUSION
Repeated imaging examinations are useful to dynamically assess the evolution of PVT in liver cirrhosis, including development, recanalization, progression, and recurrence. However, risk factors for predicting the evolution of PVT have not been sufficiently recognized yet. In future, the role of procoagulants, natural anticoagulants, and global hemostatic status indicated by thromboelastometry and thrombin generation assay for predicting the evolution of PVT in liver cirrhosis should be further considered. In addition, the impact of PVT evolution on the mortality and decompensation in patients with liver cirrhosis remains to be explored in large-scale cohort studies.
CONFLICTS OF INTEREST
Guarantor of the article: Xingshun Qi, MD.
Specific author contributions: Shixue Xu, MS, and Xiaozhong Guo, MD, contributed equally to this work. S.X. and X.Q. drafted the manuscript. All the authors provided critical revision of the manuscript for important intellectual content. All authors are responsible for the overall contents of the article.
Financial support: This work was partially supported by the Science and Technology Project Foundation of Shenyang (19-112-4-005) and Science and Technology Plan Project of Liaoning Province (2020JH2/10300163).
Potential competing interests: None to report.
ACKNOWLEDGEMENT
The authors express their gratitude to Martin Rössle (Freiburg, Germany) for his constructive comments for the improvement of the manuscript.
Contributor Information
Shixue Xu, Email: shixuexu@126.com.
Xiaozhong Guo, Email: guo_xiao_zhong@126.com.
Benqiang Yang, Email: bqyang888@sina.com.
Fernando Gomes Romeiro, Email: fernando.romeiro@unesp.br.
Massimo Primignani, Email: massimo.primignani@policlinico.mi.it.
Nahum Méndez-Sánchez, Email: nmendez@medicasur.org.mx.
Eric M. Yoshida, Email: eric.yoshida@vch.ca.
Andrea Mancuso, Email: mancandrea@libero.it.
Frank Tacke, Email: frank.tacke@charite.de.
Carlos Noronha Ferreira, Email: carlosnferreira@hotmail.com.
Valerio De Stefano, Email: valerio.destefano@unicatt.it.
REFERENCES
- 1.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;73(1):366–413. [DOI] [PubMed] [Google Scholar]
- 2.Valeriani E, Riva N, Di Nisio M, et al. Splanchnic vein thrombosis: Current perspectives. Vasc Health Risk Manag 2019;15:449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: Usefulness of screening and anticoagulation. Gut 2005;54(5):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology 2019;156(6):1582–99.e1. [DOI] [PubMed] [Google Scholar]
- 5.Mantaka A, Augoustaki A, Kouroumalis EA, et al. Portal vein thrombosis in cirrhosis: Diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol 2018;31(3):315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghabril M, Agarwal S, Lacerda M, et al. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: Analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation 2016;100(1):126–33. [DOI] [PubMed] [Google Scholar]
- 7.John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013;12(6):952–8. [PubMed] [Google Scholar]
- 8.Berry K, Taylor J, Liou IW, et al. Portal vein thrombosis is not associated with increased mortality among patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13(3):585–93. [DOI] [PubMed] [Google Scholar]
- 9.Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl 2010;16(1):83–90. [DOI] [PubMed] [Google Scholar]
- 10.Girleanu I, Stanciu C, Cojocariu C, et al. Natural course of nonmalignant partial portal vein thrombosis in cirrhotic patients. Saudi J Gastroenterol 2014;20(5):288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luca A, Caruso S, Milazzo M, et al. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 2012;265(1):124–32. [DOI] [PubMed] [Google Scholar]
- 12.Stotts MJ, Wentworth BJ, Northup PG. Management of portal vein thrombosis in cirrhosis. Semin Liver Dis 2021;41(1):79–86. [DOI] [PubMed] [Google Scholar]
- 13.Nicoară-Farcău O, Soy G, Magaz M, et al. New insights into the pathogenesis, risk factors, and treatment of portal vein thrombosis in patients with cirrhosis. Semin Thromb Hemost 2020;46(6):673–81. [DOI] [PubMed] [Google Scholar]
- 14.Gatt A, Riddell A, Calvaruso V, et al. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost 2010;8(9):1994–2000. [DOI] [PubMed] [Google Scholar]
- 15.Tripodi A, Anstee QM, Sogaard KK, et al. Hypercoagulability in cirrhosis: Causes and consequences. J Thromb Haemost 2011;9(9):1713–23. [DOI] [PubMed] [Google Scholar]
- 16.Tripodi A, Primignani M, Lemma L, et al. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol 2013;59(2):265–70. [DOI] [PubMed] [Google Scholar]
- 17.Feys HB, Canciani MT, Peyvandi F, et al. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. Br J Haematol 2007;138(4):534–40. [DOI] [PubMed] [Google Scholar]
- 18.Leebeek FW, Kluft C, Knot EA, et al. A shift in balance between profibrinolytic and antifibrinolytic factors causes enhanced fibrinolysis in cirrhosis. Gastroenterology 1991;101(5):1382–90. [DOI] [PubMed] [Google Scholar]
- 19.Hollestelle MJ, Geertzen HG, Straatsburg IH, et al. Factor VIII expression in liver disease. Thromb Haemost 2004;91(2):267–75. [DOI] [PubMed] [Google Scholar]
- 20.Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006;44(1):53–61. [DOI] [PubMed] [Google Scholar]
- 21.Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol 2012;57(1):203–12. [DOI] [PubMed] [Google Scholar]
- 22.Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: Correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009;51(4):682–9. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Razik A, Mousa N, Elhelaly R, et al. De-novo portal vein thrombosis in liver cirrhosis: Risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol 2015;27(5):585–92. [DOI] [PubMed] [Google Scholar]
- 24.Stine JG, Wang J, Shah PM, et al. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case-control study. Liver Int 2018;38(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nery F, Correia S, Macedo C, et al. Nonselective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: Results of a prospective longitudinal study. Aliment Pharmacol Ther 2019;49(5):582–8. [DOI] [PubMed] [Google Scholar]
- 26.de'Angelis N, Abdalla S, Lizzi V, et al. Incidence and predictors of portal and splenic vein thrombosis after pure laparoscopic splenectomy. Surgery 2017;162(6):1219–30. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Li H, Zhang T, et al. Splanchnic vein thrombosis in liver cirrhosis after splenectomy or splenic artery embolization: A systematic review and meta-analysis. Adv Ther 2021;38(4):1904–30. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Guo X, Xu X, et al. Association of portal venous system thrombosis with endoscopic variceal treatment: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2021;32(2):125–31. [DOI] [PubMed] [Google Scholar]
- 29.Nery F, Carneiro P, Correia S, et al. Systemic inflammation as a risk factor for portal vein thrombosis in cirrhosis: A prospective longitudinal study. Eur J Gastroenterol Hepatol 2020. [Epub ahead of print November 17, 2020.] [DOI] [PubMed] [Google Scholar]
- 30.Raparelli V, Basili S, Carnevale R, et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017;65(2):571–81. [DOI] [PubMed] [Google Scholar]
- 31.Qi X, Han G, He C, et al. CT features of non-malignant portal vein thrombosis: A pictorial review. Clin Res Hepatol Gastroenterol 2012;36(6):561–8. [DOI] [PubMed] [Google Scholar]
- 32.Simonetto DA, Singal AK, Garcia-Tsao G, et al. ACG clinical guideline: Disorders of the hepatic and mesenteric circulation. Am J Gastroenterol 2020;115(1):18–40. [DOI] [PubMed] [Google Scholar]
- 33.Whitesell RT, Steenburg SD. Imaging findings of acute intravascular thrombus on non-enhanced computed tomography. Emerg Radiol 2014;21(3):271–7. [DOI] [PubMed] [Google Scholar]
- 34.Berzigotti A, García-Criado A, Darnell A, et al. Imaging in clinical decision-making for portal vein thrombosis. Nat Rev Gastroenterol Hepatol 2014;11(5):308–16. [DOI] [PubMed] [Google Scholar]
- 35.Miura K, Sugawara Y, Uchida K, et al. Adult living donor liver transplantation for patients with portal vein thrombosis: A single-center experience. Transplant Direct 2018;4(5):e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Amico G, Tarantino G, Spaggiari M, et al. Multiple ways to manage portal thrombosis during liver transplantation: Surgical techniques and outcomes. Transplant Proc 2013;45(7):2692–9. [DOI] [PubMed] [Google Scholar]
- 37.Kim JD, Choi DL, Han YS. An early single-center experience of portal vein thrombosis in living donor liver transplantation: Clinical feature, management and outcome. J Korean Surg Soc 2011;81(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29(1):62–7. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Zheng SS, Liang TB, et al. Orthotopic liver transplantation for patients with hepatocellular carcinoma complicated by portal vein tumor thrombi. Hepatobiliary Pancreat Dis Int 2004;3(3):341–4. [PubMed] [Google Scholar]
- 40.Tublin ME, Dodd GD, III, Baron RL. Benign and malignant portal vein thrombosis: Differentiation by CT characteristics. AJR Am J Roentgenol 1997;168(3):719–23. [DOI] [PubMed] [Google Scholar]
- 41.Shah ZK, McKernan MG, Hahn PF, et al. Enhancing and expansile portal vein thrombosis: Value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. AJR Am J Roentgenol 2007;188(5):1320–3. [DOI] [PubMed] [Google Scholar]
- 42.Rossi S, Ghittoni G, Ravetta V, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol 2008;18(8):1749–56. [DOI] [PubMed] [Google Scholar]
- 43.Piscaglia F, Gianstefani A, Ravaioli M, et al. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl 2010;16(5):658–67. [DOI] [PubMed] [Google Scholar]
- 44.Sorrentino P, Tarantino L, D'Angelo S, et al. Validation of an extension of the international non-invasive criteria for the diagnosis of hepatocellular carcinoma to the characterization of macroscopic portal vein thrombosis. J Gastroenterol Hepatol 2011;26(4):669–77. [DOI] [PubMed] [Google Scholar]
- 45.Sherman CB, Behr S, Dodge JL, et al. Distinguishing tumor from bland portal vein thrombus in liver transplant candidates with hepatocellular carcinoma: The A-VENA criteria. Liver Transpl 2019;25(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hepatobiliary Disease Study Group, Chinese Society of Gastroenterology, Chinese Medical Association. Consensus for management of portal vein thrombosis in liver cirrhosis (2020, Shanghai). J Dig Dis 2021;22(4):176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Zhu J, Zhang C, et al. Contrast-enhanced ultrasound for the characterization of portal vein thrombosis vs tumor-in-vein in HCC patients: A systematic review and meta-analysis. Eur Radiol 2020;30(5):2871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gawande R, Jalaeian H, Niendorf E, et al. MRI in differentiating malignant versus benign portal vein thrombosis in patients with hepatocellular carcinoma: Value of post contrast imaging with subtraction. Eur J Radiol 2019;118:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stieber AC, Zetti G, Todo S, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg 1991;213(3):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonami T, Yokoyama I, Iwatsuki S, et al. The incidence of portal vein thrombosis at liver transplantation. Hepatology 1992;16(5):1195–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Gayowski TJ, Marino IR, Doyle HR, et al. A high incidence of native portal vein thrombosis in veterans undergoing liver transplantation. J Surg Res 1996;60(2):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamieson NV. Changing perspectives in portal vein thrombosis and liver transplantation. Transplantation 2000;69(9):1772–4. [DOI] [PubMed] [Google Scholar]
- 53.Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000;69(9):1873–81. [DOI] [PubMed] [Google Scholar]
- 54.Charco R, Fuster J, Fondevila C, et al. Portal vein thrombosis in liver transplantation. Transplant Proc 2005;37(9):3904–5. [DOI] [PubMed] [Google Scholar]
- 55.Bauer J, Johnson S, Durham J, et al. The role of TIPS for portal vein patency in liver transplant patients with portal vein thrombosis. Liver Transpl 2006;12(10):544–51. [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Yan Z, Luo J, et al. Rational classification of portal vein thrombosis and its clinical significance. PLoS One 2014;9(11):e112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63(3):743–52. [DOI] [PubMed] [Google Scholar]
- 58.Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016;151(4):574–7.e3. [DOI] [PubMed] [Google Scholar]
- 59.Bhangui P, Lim C, Levesque E, et al. Novel classification of non-malignant portal vein thrombosis: A guide to surgical decision-making during liver transplantation. J Hepatol 2019;71(5):1038–50. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001;345(23):1683–8. [DOI] [PubMed] [Google Scholar]
- 61.Mancuso A. Classification of portal vein thrombosis in cirrhosis. Gastroenterology 2017;152(5):1247. [DOI] [PubMed] [Google Scholar]
- 62.Valla DC, Condat B. Portal vein thrombosis in adults: Pathophysiology, pathogenesis and management. J Hepatol 2000;32(5):865–71. [DOI] [PubMed] [Google Scholar]
- 63.De Gaetano AM, Lafortune M, Patriquin H, et al. Cavernous transformation of the portal vein: Patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol 1995;165(5):1151–5. [DOI] [PubMed] [Google Scholar]
- 64.Maruyama H, Okugawa H, Takahashi M, et al. De novo portal vein thrombosis in virus-related cirrhosis: Predictive factors and long-term outcomes. Am J Gastroenterol 2013;108(4):568–74. [DOI] [PubMed] [Google Scholar]
- 65.Noronha Ferreira C, Marinho RT, Cortez-Pinto H, et al. Incidence, predictive factors and clinical significance of development of portal vein thrombosis in cirrhosis: A prospective study. Liver Int 2019;39(8):1459–67. [DOI] [PubMed] [Google Scholar]
- 66.Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: Results of a longitudinal study. Hepatology 2015;61(2):660–7. [DOI] [PubMed] [Google Scholar]
- 67.Yi F, Guo X, Wang L, et al. Impact of spontaneous splenorenal shunt on liver volume and long-term survival of liver cirrhosis. J Gastroenterol Hepatol 2021;36(6):1694–702. [DOI] [PubMed] [Google Scholar]
- 68.Xu X, Guo X, De Stefano V, et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: A systematic review and meta-analysis. Hepatol Int 2019;13(4):468–81. [DOI] [PubMed] [Google Scholar]
- 69.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol 2016;64(1):179–202. [DOI] [PubMed] [Google Scholar]
- 70.Senzolo M, Sartori TM, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int 2012;32(6):919–27. [DOI] [PubMed] [Google Scholar]
- 71.Cui SB, Shu RH, Yan SP, et al. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol 2015;27(8):914–9. [DOI] [PubMed] [Google Scholar]
- 72.Fujiyama S, Saitoh S, Kawamura Y, et al. Portal vein thrombosis in liver cirrhosis: Incidence, management, and outcome. BMC Gastroenterol 2017;17(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwon J, Koh Y, Yu SJ, et al. Low-molecular-weight heparin treatment for portal vein thrombosis in liver cirrhosis: Efficacy and the risk of hemorrhagic complications. Thromb Res 2018;163:71–6. [DOI] [PubMed] [Google Scholar]
- 74.Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vasc Pharmacol 2019;113:86–91. [DOI] [PubMed] [Google Scholar]
- 75.Pettinari I, Vukotic R, Stefanescu H, et al. Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis. Am J Gastroenterol 2019;114(2):258–66. [DOI] [PubMed] [Google Scholar]
- 76.Zhou T, Sun X, Zhou T, et al. Efficacy and safety of nadroparin calcium-warfarin sequential anticoagulation in portal vein thrombosis in cirrhotic patients: A randomized controlled trial. Clin translational Gastroenterol 2020;11(9):e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi X, Guo X, Yoshida EM, et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med 2018;16(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung JW, Kim GH, Lee JH, et al. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: A propensity score matching analysis. Clin Mol Hepatol 2014;20(4):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2016;28(1):82–9. [DOI] [PubMed] [Google Scholar]
- 80.Acuna-Villaorduna A, Tran V, Gonzalez-Lugo JD, et al. Natural history and clinical outcomes in patients with portal vein thrombosis by etiology: A retrospective cohort study. Thromb Res 2019;174:137–40. [DOI] [PubMed] [Google Scholar]
- 81.Cai M, Zhu K, Huang W, et al. Portal vein thrombosis after partial splenic embolization in liver cirrhosis: Efficacy of anticoagulation and long-term follow-up. J Vasc Interv Radiol 2013;24(12):1808–16. [DOI] [PubMed] [Google Scholar]
- 82.Sbrancia M, Antonelli E, Bassotti G, et al. Anticoagulation therapy for non malignant portal vein thrombosis in cirrhotic patients: A safe treatment? Dig Liver Dis 2016;48(Suppl 2):e82. [Google Scholar]
- 83.Wang L, Guo X, Xu X, et al. Anticoagulation favors thrombus recanalization and survival in patients with liver cirrhosis and portal vein thrombosis: Results of a meta-analysis. Adv Ther 2020;38(1):495–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noronha Ferreira C, Reis D, Cortez-Pinto H, et al. Anticoagulation in cirrhosis and portal vein thrombosis is safe and improves prognosis in advanced cirrhosis. Dig Dis Sci 2019;64(9):2671–83. [DOI] [PubMed] [Google Scholar]
- 85.Condat B, Pessione F, Helene Denninger M, et al. Recent portal or mesenteric venous thrombosis: Increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000;32(3):466–70. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez-Castro KI, Vitale A, Fadin M, et al. A prediction model for successful anticoagulation in cirrhotic portal vein thrombosis. Eur J Gastroenterol Hepatol 2019;31(1):34–42. [DOI] [PubMed] [Google Scholar]
- 87.Delgado MG, Seijo S, Yepes I, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol 2012;10(7):776–83. [DOI] [PubMed] [Google Scholar]
- 88.Scheiner B, Stammet PR, Pokorny S, et al. Anticoagulation in non-malignant portal vein thrombosis is safe and improves hepatic function. Wien Klin Wochenschr 2018;130(13-14):446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu X, Xu S, Primignani M, et al. Nonselective β-blockers may progress the thrombosis of portal venous system in cirrhotic patients: A retrospective observational study. Adv Ther 2020;37(4):1452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amitrano L, Guardascione MA, Menchise A, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol 2010;44(6):448–51. [DOI] [PubMed] [Google Scholar]
- 91.Artaza T, Lopes M, Romero M, et al. Efficacy and safety of anticoagulation in non-malignant portal vein thrombosis in patients with liver cirrhosis. Gastroenterol Hepatol 2018;41(10):611–7. [DOI] [PubMed] [Google Scholar]
- 92.Inao M, Hirahara K, Sugawara K, et al. Usefulness of balloon-occluded retrograde obliteration (B-RTO) as a consolidation procedure after anticoagulation therapy in cirrhotic patients with portal vein thrombosis. Hepatology 2015;62(Suppl 1):935A. [Google Scholar]
- 93.Xu S, Guo X, Xu X, et al. Natural history and predictors associated with the evolution of portal venous system thrombosis in liver cirrhosis. Eur J Gastroenterol Hepatol 2021. [Epub ahead of print March 12, 2021.] [DOI] [PubMed] [Google Scholar]