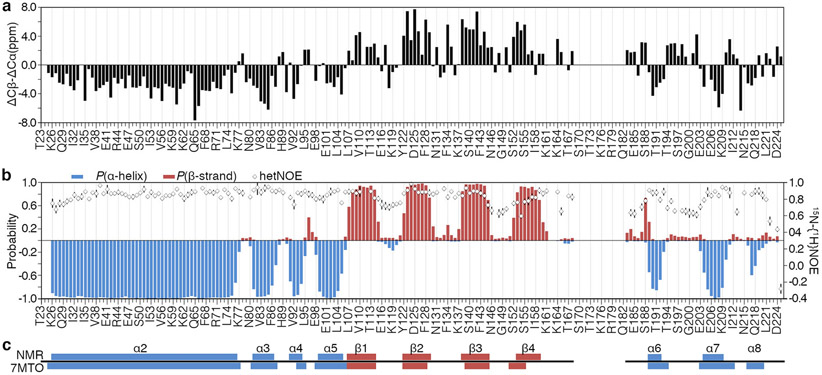
Fig. 2.
Characterization of the NAP domain (residues 23-225) of human SET/TAF-1β/I2PP2A based on NMR chemical shifts. a. Raw data depicting chemical shift deviations of Cα and Cβ carbons with respect to random coil values (Δδ(Cβ)- Δδ(Cα)) are plotted against residue numbers. Positive and negative values indicate β-strand and α-helix characters, respectively. b. The per-residue probability of secondary structure formation as predicted by Talos+, with blue bars representing α-helices and red bars indicating β-strands. Heteronuclear NOE relaxation parameters are shown as open diamonds. These data represent the average of three experiments collected with 3, 4, and 5-s delays acquired at 950 MHz, with error bars indicating one standard deviation above and below the average. c. NMR-based secondary structure predictions show good agreement with a high-resolution crystal structure of the same construct (boxes labeled α2-8 and β1-4).