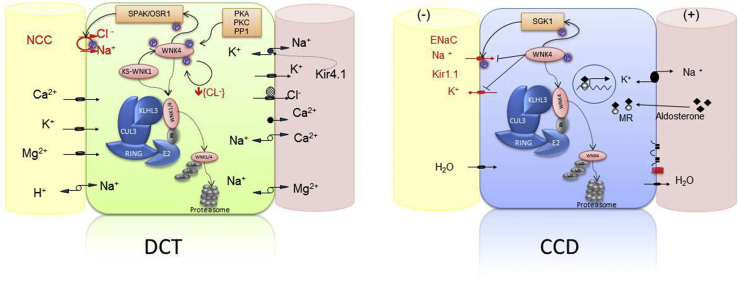
Figure 1.
Schematic representations of a cell of the distal convoluted tubule (DCT), and a principal cell of the cortical collecting duct (CCD). Inside each cell, the interaction of proteins implicated in the pathophysiology of the familial hyperkalemic hypertension is shown. In the DCT, the activity of the sodium chloride cotransporter (NCC) is maintained via a phosphorylation cascade implicating Ste20p-related Proline Alanine-rich Kinase (SPAK)/OSR/WNKs. WNK4 is active in its phosphorylated form; it can be phosphorylated by several kinases (PKC, PKA, PP1, and KS-WNK1) and is also autophosphorylated when the intracellular chloride concentration is low. Activated WNK4 phosphorylates SPAK/OSR, which, in turn, phosphorylates NCC increasing the apical NaCl reabsorption. WNK1/4 protein levels are regulated by the complex CUL3-KLHL3-E3, which targets WNK1/4 for degradation by promoting their ubiquitylation. KLHL3 binds to the acidic motif of WNK kinases through its propeller domain. Mutations in the acidic motif of WNK1/4 disrupt its interaction with KLHL3 and prevent WNK4 degradation. Mutations in KLHL3 impair either KLHL3 binding to WNK1/4 or KLHL3 binding to CUL3. Finally, mutations in CUL3 seem to modify the interaction with the COP9 signalosome enzyme, resulting in hyperneddylation and increasing ubiquitin ligase activity directed toward the substrate adaptor KLHL3. The final result is the accumulation of WNK4 with the subsequent NCC phosphorylation and increase of NaCl reabsorption. In the principal cells of the CCD, WNK4 has been implicated as a negative regulator of Na reabsorption and K secretion by epithelial sodium channel (ENaC) and renal outer medullary potassium channel (ROMK) channels, respectively.