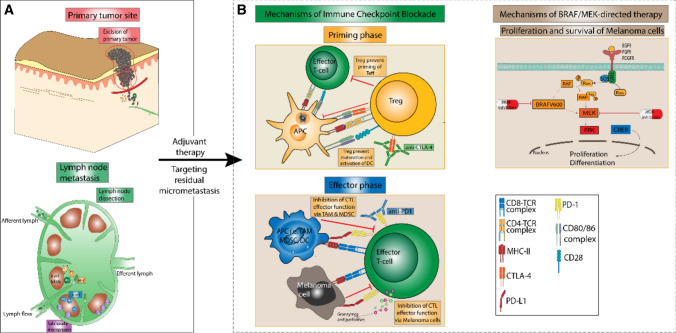
Fig. 1.
Schematic overview of the concept of adjuvant melanoma therapy and its underlying mechanisms of action. Following the initial excision of the primary tumor and if necessary existing lymph node metastasis (A), the adjuvant application of either immune-checkpoint inhibitors or BRAF/MEK-directed targeted therapy can be considered for patients with stage IIC–IIID melanoma (B). Immune-checkpoint inhibitors reinforce the anti-tumor immune response to melanoma cells both in peripheral lymph nodes and the tumor microenvironment (left panel, B). Here, the anti- cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab mainly affects the priming and activation of T cells in lymph nodes (top center). By contrast, anti- programmed cell death protein 1 (PD-1) antibodies mainly serve to restore effector cell function of T cells within the tumor microenvironment via blockade of the T-cell bound checkpoint molecule PD-1 on effector T cells (bottom center). Targeted therapy confers anti-tumor activity via the blockade of the RAF-MEK-ERK-signaling cascade, thus disrupting melanoma cell proliferation and differentiation (right panel, B). Notably, both targeted therapy and immune-checkpoint inhibitor therapy target residual melanoma cells and micrometastases, which have not been cleared by initial excision, thus reducing the risk of melanoma progression in adjuvant therapy. APC antigen-presenting cells, CTL , DC dendritic cell, MDSC , MHC II major histocompatibility complex II, PD-L1 programmed death-ligand 1, TAM , Treg regulatory T cell