Abstract
Background and purpose
As studies vary in defining the prognostic significance of smoking in acute ischaemic stroke (AIS), we aimed to determine the relation of smoking and key outcomes in patient participants who had thrombolysed AIS of the international quasi-factorial randomised Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED).
Methods
Post-hoc analyses of ENCHANTED, an international quasi-factorial randomised evaluation of intravenous alteplase-dose comparison and levels of blood pressure control in patients who had thrombolysed AIS. Multivariable logistic regression models with inverse probability of treatment weighting (IPTW) propensity scores were used to determine associations of self-reported smoking status and clinical outcomes, according to 90-day modified Rankin Scale (mRS) scores and symptomatic intracerebral haemorrhage (sICH).
Results
Of 4540 patients who had an AIS, there were 1008 (22.2%) current smokers who were younger and predominantly male, with more comorbidities of hypertension, coronary artery disease, atrial fibrillation and diabetes mellitus, and greater baseline neurological impairment, compared with non-smokers. In univariate analysis, current smokers had a higher likelihood of a favourable shift in mRS scores (OR 0.88, 95% CI 0.77 to 0.99; p=0.038) but this association reversed in a fully adjusted model with IPTW (adjusted OR 1.15, 95% CI 1.04 to 1.28; p=0.009). A similar trend was also apparent for dichotomised poor outcome (mRS scores 2–6: OR 1.18, 95% CI 1.05 to 1.33; p=0.007), but not with the risk of sICH across standard criteria.
Conclusion
Smoking predicts poor functional recovery in patients who had thrombolysed AIS.
Trial registration number
Keywords: stroke, thrombolysis
Introduction
In addition to a two-fold increased risk of acute ischaemic stroke (AIS) in the general population,1–4 cigarette smoking influences the prognosis from this illness and risk of recurrent vascular events.5–7 Intravenous alteplase has an established net benefit in patients who have AIS across a wide range of characteristics,8–11 but the interaction with smoking on recovery is controversial. Several studies suggest better outcomes in patients who had thrombolysed AIS who smoke,12 possibly by modifying platelet function,13 14 altering clot dynamics and enhancing reperfusion.15 16 However, selection bias and residual confounding limit the conclusions that can be drawn from such data.17 Recent post-hoc analyses of the efficacy and safety of MRI-based thrombolysis in wake-up stroke trial have shown that smoking does not modify the effect of intravenous thrombolysis in 486 patients who had an AIS with an unknown time of symptom onset and diffusion-weighted imaging-fluid attenuation inversion recovery mismatch on brain MRI.18 Herein, we present analyses of the international Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED) to help resolve conflicting results across studies concerning the prognostic significance of smoking in patients who had thrombolysed AIS.
Methods
Study design
ENCHANTED was an international, 2×2 partial-factorial, multicentre, prospective, randomised, open-label, blinded-endpoint trial, which evaluated the effects of low-dose (0.6 mg/kg) versus standard-dose (0.9 mg/kg) intravenous alteplase (n=3310), and intensive versus guideline-recommended blood pressure (BP) lowering (n=2227) in 4587 patients who had thrombolysis-eligible AIS.19–23
Clinical assessment and outcomes
Key demographic and clinical characteristics were recorded at the time of patient enrolment, with current smoking status obtained by self-report. Clinical outcomes were assessed at 90 days by trained investigators blind to study treatment. The primary outcome was functional status, defined by an ordinal shift in the distribution of the full range of scores on the modified Rankin Scale (mRS). Other outcomes were according to dichotomous scores on the mRS (1–6 vs 0; 2–6 vs 0–1; 3–6 vs 0–2; 4–6 vs 0–3; 5–6 vs 0–4; 6 vs 0–5), and death or neurological deterioration according to scores on the National Institutes of Health Stroke Scale (NIHSS) in 24 hours and 7 days. Safety outcomes were symptomatic intracranial haemorrhage (sICH), any ICH, any clinician reported ICH, any adjudicated ICH and any fatal ICH. The key measure of sICH was from the Safe Implementation of Thrombolysis in Stroke-Monitoring Study, defined as type 2 parenchymal ICH (>30% of the infarcted area affected by haemorrhage with mass effect or extension outside the infarct) together with either neurological deterioration (≥4 points increase in NIHSS score) or death within 24–36 hours.24 Other criteria used to further evaluate symptomatic ICH were definitions from the National Institute of Neurological Disorders and Stroke (NINDS), second and third European Cooperative Acute Stroke Studies and third International Stroke Trial.25–28
Statistical analysis
As patient characteristics were expected to differ between smokers and non-smokers, we calculated a propensity score to estimate individual probability of being a smoker based on the following baseline variables: sex, age, ethnicity (Asian vs non-Asian), systolic BP, NIHSS score, estimated premorbid mRS score (0 vs 1), presence of vascular risk factors (hypertension, coronary artery disease, other heart diseases, atrial fibrillation, diabetes mellitus or hypercholesterolaemia) and medications (anticoagulation, antiplatelet therapy, glucose lowering and lipid lowering agents). The inverse probability of treatment weighting (IPTW) adjustment for baseline imbalances29 was examined using absolute standardised differences in covariate means.30 Stabilised weights,31 used to reduce variance in the estimates of the effect of smoking, were incorporated into logistic regression models to determine associations of smoking and outcomes. Data were presented with OR and 95% CI, with a standard level of significance set at p<0.05. All analyses were undertaken using SAS software (V.9.3).
Results
Overall, 4540 patients who had thrombolysed AIS were included in these analyses, of whom 1008 (22.2%) were current smokers. Table 1 shows that compared with non-smokers, current smokers were younger, predominantly male, had more cardiovascular risk factors of hypertension, coronary artery or other heart disease, atrial fibrillation, diabetes mellitus and hypercholesterolaemia, presented with greater neurological impairment, and were more likely to have AIS with a final diagnosis of either large-vessel occlusion or cardioembolism. Time from symptom onset to alteplase administration was comparable between the two groups, but smokers were less likely to receive in-hospital nasogastric feeding, early mobilisation, compression stockings and subcutaneous heparin treatment.
Table 1.
Baseline patient characteristics and management by smoking status
| Variables | Non-smoking (N=3532) |
Smoking (N=1008) |
P value |
| Time from symptom onset to randomisation, min | 2.9 (2.2–3.7) | 2.9 (2.2–3.8) | 0.680 |
| Time from symptom onset to intravenous alteplase, min | 170 (129–217) | 175 (131–224) | 0.068 |
| Age, years | 68.2 (12.7) | 61.5 (11.2) | <0.001 |
| Female | 1583 (44.8) | 132 (13.1) | <0.001 |
| Asian | 2245 (63.6) | 282 (28.0) | <0.001 |
| Systolic blood pressure | 154 (19) | 152 (19) | <0.001 |
| Diastolic blood pressure | 86 (13) | 88 (13) | <0.001 |
| Heart rate | 79 (16) | 79 (14) | 0.549 |
| NIHSS score | 8 (5–13) | 7 (4–12) | <0.001 |
| GCS | 15 (13–15) | 15 (14–15) | <0.001 |
| Medical history | |||
| Hypertension | 2360/3532 (66.8) | 573/1008 (56.8) | <0.001 |
| Stroke | 653/3532 (18.5) | 168/1008 (16.7) | 0.185 |
| Coronary artery disease | 534/3532 (15.1) | 109/1008 (10.8) | 0.001 |
| Other heart diseases | 232/3532 (6.6) | 49/1008 (4.9) | 0.047 |
| Atrial fibrillation | 698/3528 (19.8) | 107/1008 (10.6) | <0.001 |
| Diabetes mellitus | 755/3532 (21.4) | 170/1008 (16.9) | 0.002 |
| Hypercholesterolaemia | 572/3532 (16.2) | 132/1008 (13.1) | 0.017 |
| Premorbid symptom-free (mRS 0) | 2905/3530 (82.3) | 869/1007 (86.3) | 0.003 |
| Antihypertensive agent(s) | 1698/3532 (48.1) | 373/1008 (37.0) | <0.001 |
| Statin/other lipid-lowering | 708/3529 (20.1) | 135/1007 (13.4) | <0.001 |
| Aspirin/other antiplatelet agent(s) | 831/3530 (23.5) | 153/1007 (15.2) | <0.001 |
| Warfarin anticoagulation | 90/3530 (2.5) | 10/1007 (1.0) | 0.003 |
| Glucose lowering agent(s) | 484/3530 (13.7) | 98/1007 (9.7) | 0.001 |
| Pathological subtype | |||
| Large-artery occlusion | 1377/3394 (40.6) | 427/963 (44.3) | <0.001 |
| Cardioembolism | 781/3394 (23.0) | 276/963 (28.7) | |
| Small-vessel or perforator disease | 684/3394 (20.2) | 112/963 (11.6) | |
| Other/uncertain aetiology | 552/3394 (16.3) | 148/963 (15.4) | |
| Management | |||
| Intubation and ventilation | 181/3480 (5.2) | 46/988 (4.7) | 0.491 |
| Nasogastric feeding | 636/3479 (18.3) | 153/988 (15.5) | 0.042 |
| Physiotherapy mobilisation | 1579/3479 (45.4) | 391/988 (39.6) | 0.001 |
| Compression stockings | 320/3478 (9.2) | 62/988 (6.3) | 0.004 |
| Subcutaneous heparin | 710/3532 (20.1) | 151/1008 (15.0) | <0.001 |
| Antithrombotic agent in first 24 hours | 593/3522 (16.8) | 152/1007 (15.1) | 0.188 |
| Haemicraniectomy | 34/3480 (1.0) | 13/988 (1.3) | 0.357 |
| Intensive care unit admission | 785/3479 (22.6) | 216/988 (21.9) | 0.641 |
| Rehabilitation | 1725/3480 (49.6) | 495/988 (50.1) | 0.768 |
| Decision to withdrawal active care | 97/3481 (2.8) | 14/988 (1.4) | 0.015 |
Data are n/N (%), mean (SD) or median (IQR).
GCS, Glasgow Coma Scale; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Distributions of baseline covariates were well balanced following application of propensity scores; all post-IPTW absolute standardised differences were within an acceptable margin of 0.1 (online supplemental figure S1). Although the proportional odds assumption was violated (p<0.0001), we still proceeded with an ordinal analysis for assessing the distribution of mRS scores and to compare these with analyses of dichotomised mRS scores. In univariate analysis on shift mRS scores, current smokers had a higher likelihood of a favourable outcome, compared with non-smokers (OR 0.88, 95% CI 0.77 to 0.99; p=0.038) (table 2, online supplemental figure S2). However, the direction of association was reversed in a fully adjusted model with IPTW (adjusted OR 1.15, 95% CI 1.04 to 1.28; p=0.009), indicating current smokers had an unfavourable outcome. This association with poor outcome was consistent across all dichotomised mRS scores, except for severe grades of disability (mRS scores 4–6 and 5–6).
Table 2.
Primary and secondary outcomes at 3 months
| Outcome | Non-smoking | Smoking | Univariate | Multivariable | ||
| N=3532 | N=1008 | OR (95% CI) | P value | OR (95% CI) | P value | |
| Primary outcome—ordinal mRS | 0.88 (0.77 to 0.99) | 0.038 | 1.15 (1.04 to 1.28) | 0.009* | ||
| Secondary outcome—dichotomised mRS | ||||||
| 1–6 versus 0 | 2571/3467 (74.2) | 728/981 (74.2) | 1.00 (0.85 to 1.18) | 0.973 | 1.24 (1.09 to 1.43) | 0.002 |
| 2–6 versus 0–1 | 1756/3467 (50.7) | 469/981 (47.8) | 0.89 (0.78 to 1.03) | 0.117 | 1.18 (1.05 to 1.33) | 0.007 |
| 3–6 versus 0–2 | 1265/3467 (36.5) | 319/981 (32.5) | 0.84 (0.72 to 0.98) | 0.022 | 1.18 (1.04 to 1.33) | 0.009 |
| 4–6 versus 0–3 | 875/3467 (25.2) | 187/981 (19.1) | 0.70 (0.59 to 0.83) | <0.001 | 0.98 (0.85 to 1.12) | 0.741 |
| 5–6 versus 0–4 | 532/3467 (15.3) | 111/981 (11.3) | 0.70 (0.57 to 0.88) | 0.002 | 1.01 (0.86 to 1.20) | 0.889 |
| Death | 338/3532 (9.6) | 71/1008 (7.0) | 0.72 (0.55 to 0.94) | 0.015 | 0.94 (0.77 to 1.16) | 0.586 |
| Death or neurological deterioration in 24 hours† | 305/3532 (8.6) | 87/1008 (8.6) | 1.00 (0.78 to 1.28) | 0.997 | 1.26 (1.03 to 1.54) | 0.023 |
| Death or neurological deterioration in 7 days† | 444/3532 (12.6) | 123/1008 (12.2) | 0.97 (0.78 to 1.20) | 0.757 | 1.18 (0.99 to 1.40) | 0.059 |
| Symptomatic ICH‡ | ||||||
| SITS-MOST criteria | 56/3532 (1.6) | 15/1008 (1.5) | 0.94 (0.53 to 1.67) | 0.826 | 1.17 (0.74 to 1.84) | 0.509 |
| NINDS criteria | 246/3532 (7.0) | 67/1008 (6.6) | 0.95 (0.72 to 1.26) | 0.725 | 1.29 (1.03 to 1.60) | 0.026 |
| ECASS2 criteria | 160/3532 (4.5) | 39/1008 (3.9) | 0.85 (0.59 to 1.21) | 0.367 | 1.17 (0.89 to 1.53) | 0.268 |
| ECASS3 criteria | 73/3532 (2.1) | 19/1008 (1.9) | 0.91 (0.55 to 1.52) | 0.718 | 1.01 (0.66 to 1.53) | 0.981 |
| IST3 criteria | 96/3532 (2.7) | 26/1008 (2.6) | 0.95 (0.61 to 1.47) | 0.810 | 1.06 (0.74 to 1.53) | 0.747 |
| Any ICH | 670/3532 (19.0) | 173/1008 (17.2) | 0.89 (0.74 to 1.06) | 0.193 | 1.09 (0.94 to 1.27) | 0.248 |
| Any clinical-reported ICH | 298/3532 (8.4) | 77/1008 (7.6) | 0.90 (0.69 to 1.17) | 0.417 | 1.12 (0.91 to 1.38) | 0.298 |
| Any adjudicated ICH | 593/3532 (16.8) | 155/1008 (15.4) | 0.90 (0.74 to 1.09) | 0.287 | 1.11 (0.95 to 1.30) | 0.174 |
| Fatal ICH | 36/3532 (1.0) | 8/1008 (0.8) | 0.78 (0.36 to 1.68) | 0.520 | 0.95 (0.52 to 1.73) | 0.857 |
*The common OR was estimated from an ordinal logistic-regression model and indicates the odds of a decrease of 1 in the modified Rankin Scale (mRS) score.
†Neurological deterioration (≥4 points increase in National Institutes of Health Stroke Scale (NIHSS) score) or death within 24–36 hours.
‡The main definition of symptomatic intracerebral haemorrhage (ICH) used was from Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST), as a large local or remote parenchymal intracerebral haemorrhage (>30% of the infarcted area affected by haemorrhage with mass effect or extension outside the infarct) in combination with neurological deterioration from baseline (increase of ≥4 in in the NIHSS score) or death within 36 hours. Symptomatic ICH was also assessed according to other trial criteria (see appendix).
CI, confidence interval; ECASS2 and ECASS 3, second and third European Cooperative Acute Stroke Studies; IST3, third International Stroke Study; NINDS, National Institute of Neurological Disorders and Stroke; OR, odds ratio.
svn-2020-000493supp001.pdf (849.7KB, pdf)
svn-2020-000493supp002.pdf (473.9KB, pdf)
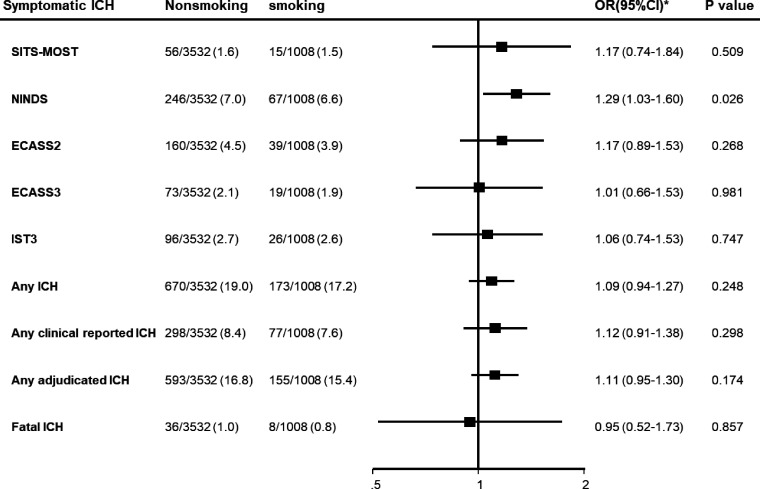
There was no significant association between smoking and different definitions of sICH, except for NINDS criteria (OR 1.29, 95% CI 1.03 to 1.60; p=0.003) (table 2, figure 1). Sensitivity analysis undertaken to explore potential confounders indicated age, sex and baseline NIHSS were the key factors influencing the direction of association (table 3); their exclusion from models produced comparable direction and magnitude of association between smoking and functional outcomes seen in univariate analysis (OR 0.96, 95% CI 0.85 to 1.09; p=0.557).
Figure 1.
Forest plot for symptomatic intracerebral haemorrhage (ICH) variables at 90 days. ECASS2/3, second and third European Cooperative Acute Stroke Studies; IST3, third International Stroke Trial; NINDS, National Institute of Neurological Disorders and Stroke; SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study.
Table 3.
Logistic regression models for primary outcome, with variable exclusions
| Outcome | Models | OR (95% CI) | P value |
| Ordinal mRS | Model 1 | 1.23 (1.07 to 1.40) | 0.003 |
| Model 2 | 1.26 (1.10 to 1.43) | 0.001 | |
| Model 3 | 1.12 (0.98 to 1.27) | 0.088 | |
| Model 4 | 0.96 (0.85 to 1.09) | 0.557 |
Model 1: fully adjusted for sex, age, ethnic group, baseline National Institutes of Health Stroke Scale (NIHSS), baseline systolic blood pressure, history of hypertension, acute coronary syndrome, other heart disease, diabetes mellitus, hypercholesterolaemia, prior use of antiplatelet use, anticoagulant use, glucose lowering agent, lipid lowering agent, modified Rankin Scale (mRS) before stroke.
Model 2: variables in model 1 with exclusion of sex.
Model 3: variables in model 1 with exclusion of age and sex.
Model 4: variables in model 1 with exclusion of age, sex and baseline NIHSS score.
Discussion
In these secondary analyses of the large ENCHANTED database, we have shown that smokers had a poor functional outcome after treatment with intravenous thrombolysis for AIS. The adverse outcome was also reflected in greater odds of early neurological deterioration, but there was no clear association of smoking and sICH. The discordant results across the other studies on this topic may relate to incomplete adjustment for confounding variables, in particular neurological severity.
The finding that smokers were younger and had more cardiovascular risk factors than non-smokers with AIS, and in having a greater likelihood of large-vessel occlusion or cardioembolism, is consistent with other studies,7 32 suggesting an acceleration of atherosclerosis and thrombus formation from smoking.33–37 However, the so-called ‘smoking-thrombolysis paradox’, promoted in relation to a potential increase in the efficacy of thrombolysis in smokers,16 37 38 may have been influenced by systematic errors and/or residual confounding,17 particularly in relation to neurological severity, as we have shown. A large (n=10 825) multicentre prospective study of AIS has also shown that current and recent smoking was associated with unfavourable functional outcome,7 while a Taiwanese registry study found that smokers had twofold greater mortality and prolonged disability after stroke.38 These findings support our findings where we used a propensity score approach to adjust covariate confounders between smokers and non-smokers.
Several potential mechanisms could explain the poor prognosis in patients who had thrombolysed AIS who smoke. Smoking may compromise recovery due to abnormal cardiopulmonary function,6 7 while also specific adverse effects on the vascular endothelium that could inhibit restorative processes in the brain.39 An increase in haematocrit may potentially increase resistance to blood flow and oxygen supply.40 Further imaging studies defining the relation of smoking and post-thrombolysis recanalisation status may clarify such mechanistic processes.
Key strengths of this study include the use of data derived from an international, multicentre, study, which had a rigorous protocol, standardised data collection procedures, and objective outcome measures. The large sample size and use of multivariable models with propensity score matching adjustment of known covariates offered an advantage of reducing the influence of confounding. We recognise, however, that the inclusion of clinical trial participants with predominantly mild-to-moderate AIS from Asia may raise concerns over the generalisability of these results. While other studies have shown a dose-dependent pattern of smoking,41 42 we were limited in only being able to use a simple binary measure of this exposure without any data on the frequency, duration and time from cessation of smoking. Finally, as these analyses were not prespecified, they are prone to random error and residual confounding.
In summary, our study has shown that smokers adversely influence functional recovery in patients who had thrombolysed AIS, compared with non-smokers.
Acknowledgments
Dr Wang is supported by grants from the National Heart Foundation (102117) and New South Wales Health. Dr Yang is supported by grants from National Natural Science Foundation of China (81870940). Dr Robinson is a National Institute for Health Research Senior Investigator. Dr Anderson holds a Senior Investigator Fellowship of the National Health and Medical Research Council of Australia.
Footnotes
Contributors: CA contributed to study design, organisation, execution, statistical review and review and critique of the report. LS1 (L Sun) contributed to study execution and writing of the report. LS2 (L Song) contributed to study design, review and critique of the report. XW contributed to study design, data analysis and review and critique of the report. JY, RIL, TR, PML, HA, BO and JC contributed to study organisation, execution and review and critique of the report.
Funding: This study is funded by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant 1020462), the Stroke Association of the United Kingdom (Reference TSA 2012/01), and the National Council for Scientific and Technological Development of Brazil (CNPq grant number 467322/2014-7).
Disclaimer: The views expressed in the article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care.
Competing interests: RTL reports personal fees from Covidien and Pfizer; HA reports lecture fees from Takeda, Daiichi Sankyo, Astellas, and Aska Pharmaceuticals; outside the submitted work; JC reports research grants and lecture fees from Servier for the ADVANCE trial and post-trial follow-up; BO reports receiving fees for service on the data and safety monitoring committee of the THALES (ticagrelor) trial. CA reports personal lecture fees and travel support, and grants paid to his institution, from Takeda China.
Data availability statement
Data are available upon reasonable request. Individual deidentified participant data used in these analyses can be shared by formal request with protocol and statistical analysis plan from any qualified investigator to the Research Office of The George Institute for Global Health, Australia. A tailored dataset specific to the research question will be shared for 6 months, and the data can be only accessed by qualified statisticians for the proposed analysis.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Every ethics committee at the participating centers. The study protocol was approved by the appropriate Ethics Committee at each participating hospital, and written informed consent was obtained from each patient or an appropriate surrogate.
References
- 1.Markidan J, Cole JW, Cronin CA, et al. Smoking and risk of ischemic stroke in young men. Stroke 2018;49:1276–8. 10.1161/STROKEAHA.117.018859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueshima H, Choudhury SR, Okayama A, et al. Cigarette smoking as a risk factor for stroke death in Japan: nippon DATA80. Stroke 2004;35:1836–41. 10.1161/01.STR.0000131747.84423.74 [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, D'Agostino RB, Kannel WB. Cigarette smoking as a risk factor for stroke. JAMA 1988;259:1025–9. 10.1001/jama.1988.03720070025028 [DOI] [PubMed] [Google Scholar]
- 4.Ding N, Sang Y, Chen J, et al. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J Am Coll Cardiol 2019;74:498–507. 10.1016/j.jacc.2019.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang JL, Morris JK, Wald NJ, et al. Mortality in relation to TAR yield of cigarettes: a prospective study of four cohorts. BMJ 1995;311:1530–3. 10.1136/bmj.311.7019.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ovbiagele B, Weir CJ, Saver JL, et al. Effect of smoking status on outcome after acute ischemic stroke. Cerebrovasc Dis 2006;21:260–5. 10.1159/000091224 [DOI] [PubMed] [Google Scholar]
- 7.Matsuo R, Ago T, Kiyuna F, et al. Smoking status and functional outcomes after acute ischemic stroke. Stroke 2020;51:846–52. 10.1161/STROKEAHA.119.027230 [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Emberson J, Blackwell L, et al. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke 2016;47:2373–9. 10.1161/STROKEAHA.116.013644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 10.Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke Study Group. N Engl J Med 1999;340:1781–7. 10.1056/NEJM199906103402302 [DOI] [PubMed] [Google Scholar]
- 11.IST-3 collaborative group . Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third International Stroke Trial [IST-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol 2013;12:768–76. 10.1016/S1474-4422(13)70130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ovbiagele B, Saver JL. The smoking-thrombolysis paradox and acute ischemic stroke. Neurology 2005;65:293–5. 10.1212/01.WNL.0000168163.72351.f3 [DOI] [PubMed] [Google Scholar]
- 13.Ovbiagele B, Wang J, Johnston SC, et al. Effect of clopidogrel by smoking status on secondary stroke prevention. Circulation 2017;135:315–6. 10.1161/CIRCULATIONAHA.116.024957 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Wang Y, Song H, et al. Clopidogrel and ischemic stroke outcomes by smoking status: smoker's paradox? J Neurol Sci 2017;373:41–4. 10.1016/j.jns.2016.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali SF, Smith EE, Bhatt DL, et al. Paradoxical association of smoking with in-hospital mortality among patients admitted with acute ischemic stroke. J Am Heart Assoc 2013;2:e000171. 10.1161/JAHA.113.000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kufner A, Nolte CH, Galinovic I, et al. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke 2013;44:407–13. 10.1161/STROKEAHA.112.662148 [DOI] [PubMed] [Google Scholar]
- 17.Aune E, Røislien J, Mathisen M, et al. The "smoker's paradox" in patients with acute coronary syndrome: a systematic review. BMC Med 2011;9:97. 10.1186/1741-7015-9-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlemm L, Kufner A, Boutitie F, et al. Current Smoking Does Not Modify the Treatment Effect of Intravenous Thrombolysis in Acute Ischemic Stroke Patients-A Post-hoc Analysis of the WAKE-UP Trial. Front Neurol 2019;10:1239. 10.3389/fneur.2019.01239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson CS, Woodward M, Arima H, et al. Statistical analysis plan for evaluating low- vs. standard-dose alteplase in the enhanced control of hypertension and thrombolysis strokE stuDy (enchanted). Int J Stroke 2015;10:1313–5. 10.1111/ijs.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Sharma VK, Robinson T, et al. Rationale, design, and progress of the ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy (ENCHANTED) trial: An international multicenter 2 × 2 quasi-factorial randomized controlled trial of low- vs. standard-dose rt-PA and early intensive vs. guideline-recommended blood pressure lowering in patients with acute ischaemic stroke eligible for thrombolysis treatment. Int J Stroke 2015;10:778–88. 10.1111/ijs.12486 [DOI] [PubMed] [Google Scholar]
- 21.Anderson CS, Robinson T, Lindley RI, et al. Low-Dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med 2016;374:2313–23. 10.1056/NEJMoa1515510 [DOI] [PubMed] [Google Scholar]
- 22.Anderson CS, Huang Y, Lindley RI, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet 2019;393:877–88. 10.1016/S0140-6736(19)30038-8 [DOI] [PubMed] [Google Scholar]
- 23.Anderson CS, Woodward M, Arima H, et al. Statistical analysis plan for evaluating different intensities of blood pressure control in the enhanced control of hypertension and thrombolysis strokE stuDy. Int J Stroke 2019;14:555–8. 10.1177/1747493018806170 [DOI] [PubMed] [Google Scholar]
- 24.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in Stroke-Monitoring study (SITS-MOST): an observational study. Lancet 2007;369:275–82. 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 25.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–8. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 26.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). second European-Australasian acute stroke study Investigators. Lancet 1998;352:1245–51. 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 27.IST-3 collaborative group, Sandercock P, Wardlaw JM, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–63. 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 29.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 30.Haviland A, Nagin DS, Rosenbaum PR. Combining propensity score matching and group-based trajectory analysis in an observational study. Psychol Methods 2007;12:247–67. 10.1037/1082-989X.12.3.247 [DOI] [PubMed] [Google Scholar]
- 31.Rheta E, Lanehart PRdG ESK, Bellara AP, et al. Propensity score analysis and assessment of propensity score approaches using SAS procedures [online]. Available: http://supportsascom/resources/papers/proceedings12/314-2012pdf
- 32.Ntaios G, Milionis H, Vemmos K, et al. Small-Vessel occlusion versus large-artery atherosclerotic strokes in diabetics: patient characteristics, outcomes, and predictors of stroke mechanism. Eur Stroke J 2016;1:108–13. 10.1177/2396987316647856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Y, Ye D, Wu DJ, et al. Role of cigarette smoking in the development of ischemic stroke and its subtypes: a Mendelian randomization study. Clin Epidemiol 2019;11:725–31. 10.2147/CLEP.S215933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji R, Pan Y, Yan H, et al. Current smoking is associated with extracranial carotid atherosclerotic stenosis but not with intracranial large artery disease. BMC Neurol 2017;17:120. 10.1186/s12883-017-0873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson SC, Burgess S, Michaëlsson K. Smoking and stroke: a Mendelian randomization study. Ann Neurol 2019;86:468–71. 10.1002/ana.25534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barua RS, Sy F, Srikanth S, et al. Effects of cigarette smoke exposure on clot dynamics and fibrin structure: an ex vivo investigation. Arterioscler Thromb Vasc Biol 2010;30:75–9. 10.1161/ATVBAHA.109.195024 [DOI] [PubMed] [Google Scholar]
- 37.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–15. 10.1161/ATVBAHA.113.300156 [DOI] [PubMed] [Google Scholar]
- 38.Wang H-K, Huang C-Y, Sun Y-T. Smoking paradox in stroke survivors?: uncovering the truth by interpreting 2 sets of data. Stroke 2020;51:STROKEAHA119027012. 10.1161/STROKEAHA.119.027012 [DOI] [PubMed] [Google Scholar]
- 39.Rogers RL, Meyer JS, Shaw TG, et al. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. JAMA 1983;250:2796–800. 10.1001/jama.1983.03340200030024 [DOI] [PubMed] [Google Scholar]
- 40.Li B, Li D, Liu J-F. “Smoking paradox” is not true in patients with ischemic stroke: a systematic review and meta-analysis. J Neurol 2019. 10.1007/s00415-019-09596-3. [Epub ahead of print: 29 Oct 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 2015;386:1447–56. 10.1016/S0140-6736(15)00340-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein KA, Viscoli CM, Spence JD, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology 2017;89:1723–9. 10.1212/WNL.0000000000004524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000493supp001.pdf (849.7KB, pdf)
svn-2020-000493supp002.pdf (473.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Individual deidentified participant data used in these analyses can be shared by formal request with protocol and statistical analysis plan from any qualified investigator to the Research Office of The George Institute for Global Health, Australia. A tailored dataset specific to the research question will be shared for 6 months, and the data can be only accessed by qualified statisticians for the proposed analysis.