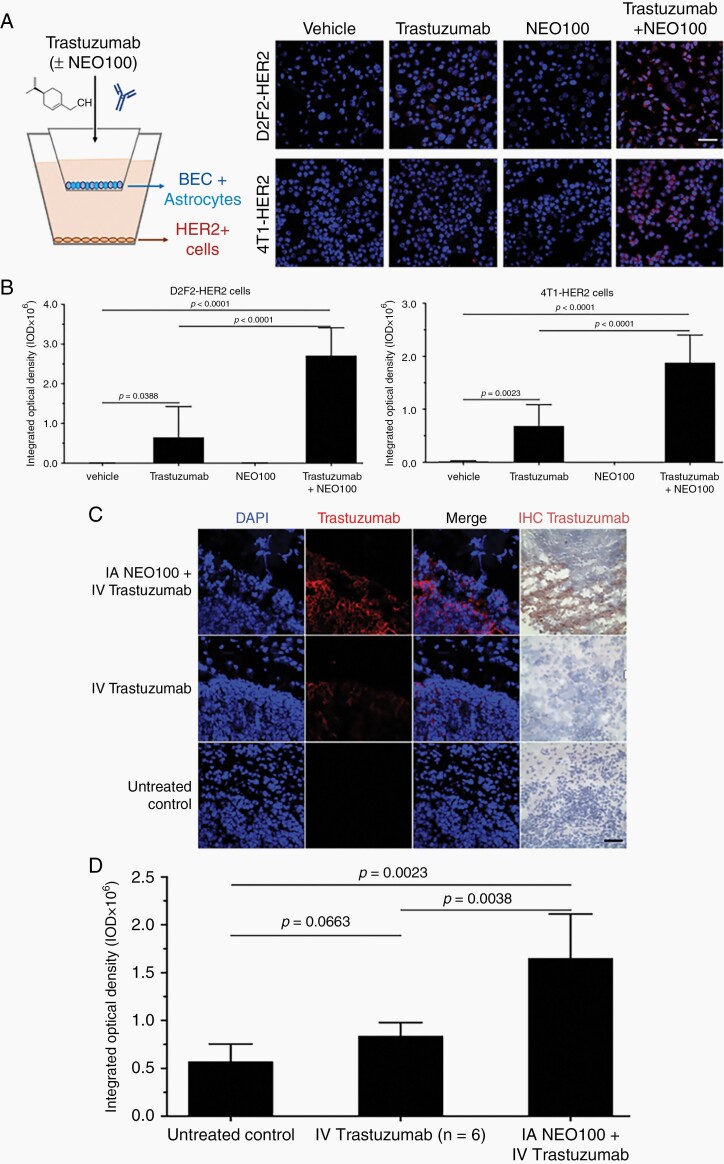
Fig. 1.
NEO100 facilitates blood-brain barrier (BBB) penetration of trastuzumab. (A) Left: Illustration of in vitro Transwell BBB set up. Brain endothelial cells (BEC) + astrocytes formed the BBB, whereas HER2+ cells at the bottom represented the target for trastuzumab binding. Right panels: Representative confocal pictures of 4T1-HER2+ and D2F2-HER2+ cells after immunostaining with red fluorophore-conjugated anti-human IgG antibody (detecting trastuzumab). As indicated, the top chambers received vehicle, trastuzumab, NEO100, or a combination of trastuzumab + NEO100. Concentration of NEO100 was 1 mM, which was confirmed to be non-cytotoxic (not shown). After 10 min, HER2+ cells were removed and stained. Red fluorescent staining denotes trastuzumab bound to cells; blue staining (DAPI) identifies nuclei. Scale bar, 50 µm. (B) Red fluorescent staining was quantitated and presented in bar graphs, representing the average of three independent experiments performed in triplicate. Left: D2F2-HER2+ cells; right: 4T1-HER2+ cells. (C) Balb/c mice bearing intracranial 4T1-HER2+ cells were treated with intra-arterial (IA) NEO100 (or vehicle), immediately followed by intravenous (IV) trastuzumab, or remained untreated (control). Twenty-four hours later, perfused brains were collected and sections were subjected to immunostaining with a biotinylated antibody recognizing trastuzumab (right panel; brown stain), or a fluorescently labeled antibody recognizing human IgG (second panel from left; red stain). DAPI was used as the counterstain. Representative confocal images are shown. (Brain sections from mice treated with NEO100 alone are not shown, as they yielded results identical to untreated control.). Scale bar, 50 µm. (D) Red fluorescent staining (indicating cell-bound trastuzumab) from C was quantitated and presented in bar graphs, representing the averages from multiple independent images.