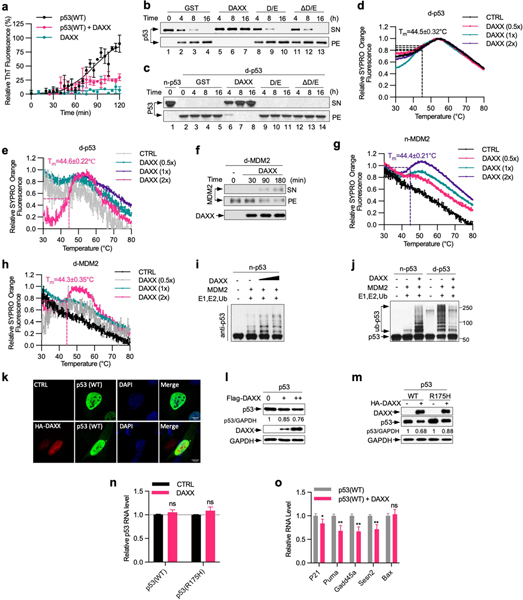
Extended Data Fig. 7 |. DAXX maintains the native conformation of both p53 and MDM2.
a, DAXX abrogates p53 fibrillization. Recombinant wild-type p53 and DAXX–6xHis proteins (5 μM each) were incubated alone or together at 37 °C for 2 h in the presence of ThT (25 μM). Formation of amyloid fibrils was assayed by ThT. b, c, DAXX(ΔD/E) and DAXX(D/E) cannot protect p53 from aggregation. Native p53 (n-p53) (b), or denatured p53 aggregates (d-p53) (c), (100 nM each) was incubated with GST, Flag–DAXX, DAXX(D/E) or DAXX(ΔD/E) (200 nM each) at 37 °C (b) or at 25 °C (c) for the indicated times. Samples were partitioned into supernatant (soluble) and pellet (insoluble) fractions via sedimentation, and analysed by western blot. As in Fig. 4a, b except that DAXX(D/E) and DAXX(ΔD/E) samples are included. d, e, g, h, DAXX restore native conformation to denatured p53 and MDM2. Native p53 (d) or native MDM2 (n-MDM2, g), or denatured p53 (e) or MDM2 (d-MDM2, h), (1 μM each) was incubated alone or together with GST or DAXX–6xHis (0.5, 1, 2 μM, from Sf9 cells) at the indicated molar ratios for 3 h and analysed by thermal shift assay. The transition of the unfolding curve represents the temperature at which the protein unfolding occurs (Tm). f, DAXX dissolve preformed MDM2 aggregates. d-MDM2 (100 nM) was incubated with Flag–DAXX (200 nM) at 25 °C for the indicated times. Supernatant (soluble) and pellet (insoluble) fractions after sedimentation were analysed by western blot. i, DAXX enhances MDM2-mediated p53 ubiquitination. Native p53 (20 nM) was incubated with native MDM2 (45 nM) in the presence or absence of DAXX (20 or 100 nM) at 37 °C for 1.5 h. E1, E2 and His-ubiquitin (His-Ub) were then added for in vitro ubiquitination assay. The reaction mixtures were analysed by western blot. j, Native MDM2-mediated ubiquitination of native p53 (20 nM) in the presence or absence of Flag–DAXX (100 nM), or of denatured p53 (20 nM) pre-incubated with or without Flag–DAXX (100 nM) for 3 h at 25 °C. k, l, DAXX reduces p53 levels in cells, but does not alter the largely diffuse nuclear localization pattern of p53. Flag–p53 was transfected into U2OS cells together with empty vector or DAXX. Cells were analysed by immunofluorescence (k) and western blot (l). m–o, H1299 cells inducibly expressing wild-type p53 or p53(R175H) were transfected with control vector (−) or HA–DAXX. Upon induction of p53 expression by Dox (1 μg ml−1), cells were analysed for protein levels by western blot with relative p53/GAPDH ratios indicated (m) and for mRNA levels of p53 (n) and p53 target genes (o) by qRT–PCR. Scale bar, 10 μm. Assays in panels have been performed two (d, e, g, h, k–m) or three (b, c, f, i, j) times with similar results. Numerical data are mean ± s.d. (n = 3) and are representative of two independent experiments (a, n, o). *P < 0.05, **P < 0.01, ns, not significant; unpaired Student’s t-test.