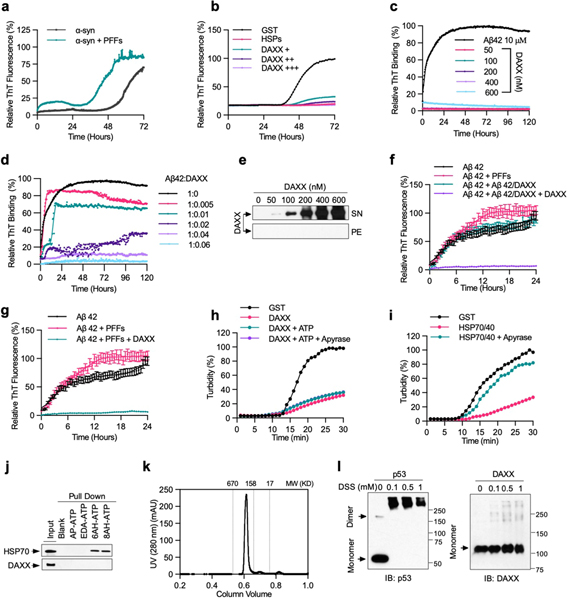
Extended Data Fig. 2 |. DAXX prevents α-Syn and Aβ42 aggregation, acts independently of ATP, and probably exists as a monomer.
a, b, PFF-induced aggregation of soluble α-Syn monomers and its inhibition by DAXX. α-Syn monomers (13.3 μM) was incubated alone, together with in α-Syn PFFs (133 nM) (a), or together with in α-Syn PFFs (133 nM) in the presence of GST (0.2 μM), HSPs (0.2 μM HSP70, 0.1 μM HSP40, and 0.4 μM HSP104(A503S)), and DAXX–6xHis (from Sf9 cells, 0.1, 0.2,and 0.4 μM) (b). Aggregation was monitored by real-time quaking-induced conversion (RT-QuIC) assay. c–e, DAXX suppresses Aβ42 fibrillization for a prolonged incubation, during which DAXX itself did not form fibrils or other sedimentable aggregates. Aβ42 monomers (10 μM) and DAXX–6xHis (from Sf9 cells, 0.05, 0.1, 0.2, 0.4 and 0.6 μM) were incubated alone (c, e) or together (d) at 37 °C for 120 h. Formation of fibrils was analysed by ThT fluorescence assay (c, d). Solubility of DAXX was analysed by sedimentation assay (e). f, g, DAXX blocks Aβ42 monomers to form PFFs that accelerate aggregation of fresh Aβ42 monomers and Aβ42 PFF-induced aggregation of fresh Aβ42 monomers. Aβ42 monomers (10 μM) were incubated at 37 °C alone, together Aβ42 PFFs (6 nM) (f, g), Aβ42 (6 nM) preincubated with DAXX–6xHis (from Sf9 cells) at a 100:1 molar ratio (Aβ42/DAXX), Aβ42/DAXX plus DAXX–6xHis (0.6 μM) (DAXX) (f), or Aβ42 PFFs (6 nM) in the presence of DAXX (g). Formation of fibrils was analysed by ThT fluorescence assay. Assays in f and g were done at the same time. h, i, The chaperone activity of DAXX is not affected by the addition of ATP or the treatment of apyrase. Luciferase (0.2 μM) was heated at 42 °C in presence of GST (0.2 μM) (h, i), DAXX–6xHis (insect cells, 0.2 μM) with or without ATP (5 mM ATP-Mg2+ plus an ATP-regeneration system) and apyrase as indicated (h), or HSP70–HSP40 (0.2 and 0.1 μM, respectively) with or without apyrase (i). Aggregation formation was monitored by OD at 600 nm. j, DAXX does not bind to ATP. Recombinant DAXX–6xHis and HSP70 were incubated with agarose beads conjugated without ATP (−) or with ATP via the phosphate moiety (AP-ATP), ribose moiety (EDA-ATP), or the adenine base at different positions (6AH-ATP and 8AH-ATP). The input and pulldown samples were analysed by western blot. k, DAXX exists as a homogeneous species of relatively low molecular weights. Recombinant Flag–DAXX protein was analysed by Superdex 200 10/300 GL column. Proteins standards (in kDa) are indicated. l, DAXX likely exists as a monomer. Recombinant Flag–DAXX (1 μM) was crosslinked with indicated concentration of DSS at 25 °C for 30 min and analysed by western blot. Flag–p53 (1 μM), which is expected to be a tetramer, was used as control. Similar results were obtained for DAXX–6xHis. Assays have been performed three (b–e, k, l) or two (a, h, i, j) times with similar results. Numerical data are mean ± s.d. (n = 3) and are representative of three independent experiments (f, g).