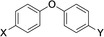
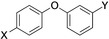
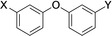
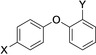
Table 1.
Compounds screened for their general inhibitory potential towards PARP10 in vitro and in cells.
|
Comp. |
X |
Y |
PARP10cat[a] Automod. |
CFA[b] |
|---|---|---|---|---|
|
|
|
|
|
|
|
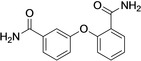
4 |
C(O)NH2 |
C(O)OH |
active |
inactive |
|
5 |
C(O)NH2 |
C(O)OMe |
active |
5‐10 μm |
|
6 |
C(S)NH2 |
C(O)OMe |
inactive |
inactive |
|
7 |
CN |
C(O)NHNH2 |
n.a.[c] |
inactive |
|
8 |
C(O)NH2 |
C(O)NHNH2 |
active |
inactive |
|
9 |
C(S)NH2 |
C(O)OH |
active |
inactive |
|
10 |
C(O)NH2 |
CN |
active |
1–2 μm |
|
11 |
C(O)NH2 |
5‐tetrazolyl |
active |
inactive |
|
12 |
C(S)NH2 |
5‐tetrazolyl |
active |
inactive |
|
13 |
C(S)NH2 |
CN |
active |
inactive |
|
14 |
C(O)OMe |
5‐tetrazolyl |
inactive |
inactive |
|
15 |
C(O)OH |
5‐tetrazolyl |
inactive |
inactive |
|
16 |
CN |
CN |
active |
inactive |
|
17 |
C(S)NH2 |
C(S)NH2 |
inactive |
inactive |
|
18 |
C(O)NH2 |
C(S)NH2 |
active |
5–10 μm |
|
|
|
|
|
|
|
19 |
CN |
C(O)NH2 |
active |
5–10 μm [d] |
|
20 |
C(O)NH2 |
C(O)NH2 |
active |
1–2 μm |
|
21 |
C(O)NH2 |
C(O)OH |
inactive |
inactive |
|
22 |
C(O)NH2 |
C(O)OMe |
inactive |
inactive |
|
23 |
5‐tetrazolyl |
C(O)NH2 |
active |
inactive |
|
24 |
C(S)NH2 |
C(O)NH2 |
active |
inactive |
|
25 |
C(O)NH2 |
CN |
active |
inactive |
|
26 |
C(O)NH2 |
5‐tetrazolyl |
active |
inactive |
|
27 |
C(O)NH2 |
C(S)NH2 |
active |
inactive |
|
|
|
|
|
|
|
28 |
CN |
CN |
inactive |
n.a. |
|
29 |
C(O)NH2 |
C(O)NH2 |
inactive |
inactive |
|
|
|
|
|
|
|
30 |
C(O)NH2 |
C(O)NH2 |
inactive |
n.a. |
|
31 |
CN |
C(O)NH2 |
inactive |
n.a. |
|
32 |
C(O)NH2 |
C(O)OH |
inactive |
n.a. |
|
|
|
|||
[a] Automodification and analysis by SDS‐PAGE, active and inactive refers to compounds that inhibited at 10 μm or did not, respectively. Examples are shown in Figures 3 and S1 in the Supporting Information.[b] Colony‐formation assay (CFA) in HeLa‐PARP10 cells with estimated IC50 values. [c] not analyzed (n.a.). [d] Very small colonies, suggesting some toxic effect.