Abstract
Systematic discontinuation of long‐term treatment with nucleos(t)ide analogues (NAs) is one strategy to increase functional cure rates in patients with chronic hepatitis B e antigen (HBeAg)–negative hepatitis B. Currently, available study results are heterogeneous; however, long‐term hepatitis B surface antigen (HBsAg) loss rates of up to 20% have been reported in prospective trials. This review proposes criteria that can be used when considering NA discontinuation in patients with chronic hepatitis B virus (HBV). Discontinuing NA treatment frequently results in a virologic and biochemical relapse that runs through different phases: the lag phase, reactivation phase, and consolidation phase. The HBV‐DNA flares observed during the reactivation phase are often transient and most likely represent a trigger for inducing a long‐term immune control by specific CD8+ T cells, and therefore do not need immediate interventions but close follow‐up evaluation. Low HBsAg levels at the time of treatment cessation predict a positive long‐term response to NA discontinuation associated with a higher likelihood of HBsAg clearance. Other host and viral biomarkers are currently under evaluation that may prove to be helpful to further characterize the population that may benefit most from the finite NA treatment concept. Potential harmful biochemical flares during the reactivation phase need to be identified early and can be effectively terminated by reintroducing NA treatment. Hepatic decompensation represents a risk to patients with cirrhosis undergoing NA discontinuation. Therefore, the finite NA approach should only be considered after excluding advanced fibrosis and cirrhosis and if a close follow‐up of the patient and supervision by an experienced physician can be guaranteed. Conclusion: For selected patients, NA discontinuation has become a powerful tool to achieve control over HBeAg‐negative HBV infections. Its significant effect represents a challenge to novel treatment approaches, but it may also serve as their enhancer.
Abbreviations
- ALT
alanine aminotransferase
- ETV
entecavir
- HBcrAg
hepatitis B core‐related antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- LdT
telbivudine
- LMV
lamivudine
- NA
nucleos(t)ide analogue
- PD‐L1
programmed cell death 1 ligand 1
- PD‐1
programmed cell death 1 protein
- TDF
tenofovir disoproxil fumarate
- TLR
toll‐like receptor
- ULN
upper limit of normal
Chronic hepatitis B virus (HBV) infection is a major global health concern. Approximately 250 million people are chronically infected with the virus, putting them at risk of serious liver disease, including cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).( 1 ) Nucleos(t)ide analogue (NA) treatments for chronic hepatitis B are available, which provide sustained suppression of HBV replication, thus slowing or preventing disease progression.( 1, 2, 3 ) Endpoints for treatment with NA are stages of stable immune control over the infection, which are characterized by either undetectable hepatitis B e antigen (HBeAg) and the appearance of antibody to hepatitis B e antigen (anti‐HBe) in formerly HBeAg‐positive patients or undetectable HBsAg, which is considered a functional cure of HBV infections.( 4 ) However, hepatitis B surface antigen (HBsAg) loss was observed in no more than 10% of HBeAg‐positive patients and, more disappointingly, in less than 1% of HBeAg‐negative patients after 8 years of tenofovir disoproxil fumarate (TDF) treatment.( 5 ) As a result, patients with HBeAg‐negative chronic hepatitis B are almost always considered to require lifelong NA treatment.( 6, 7, 8 )
According to current guidelines, NA treatment may be discontinued in patients with HBeAg‐positive disease who achieve HBeAg loss, seroconversion to anti‐HBe, and undetectable HBV DNA. After achieving such response, patients receive 12 months of consolidation therapy.( 6, 7, 8 ) In addition, some recent guidelines include the possibility of discontinuing long‐term NA treatment in HBeAg‐negative patients, even if HBsAg levels are still detectable.( 6, 8 ) The reason for this recommendation is that withdrawal of NA treatment in this population was shown to trigger immune control in some patients, resulting in a significant number of patients achieving HBsAg loss in some trials. However, despite these encouraging results, there is no general consensus on the value of NA discontinuation and how it can be included in an individualized treatment approach. By reviewing the current literature, this article aims to summarize the current knowledge of this finite treatment concept in the HBeAg‐negative patient population.
The Concept of NA Withdrawal as an Approach to Achieve a Functional Cure
Immune control of HBV infections relies on a complex interplay between the virus and host immunity. There is a consensus that adaptive immune responses, mediated especially by virus‐specific CD4+ and CD8+ T cells, play a key role in the defense against HBV infection.( 9 ) Accordingly, chronic HBV infection is characterized by undetectable or weak HBV‐specific CD8+ T‐cell responses.( 10, 11 ) This so‐called T‐cell exhaustion is believed to be the result of a chronic antigen overstimulation of HBV‐specific CD8+ T cells, which gradually leads to a loss of antiviral effector functions.( 11, 12, 13 ) This overstimulation with viral proteins such as HBeAg or HBsAg, which are excessively produced during the HBV infection, can be considered a viral strategy to circumvent the activation of the host antiviral responses and to establish chronic infection.( 14, 15 )
T‐cell exhaustion in chronic HBV infections is further characterized by sustained expression of multiple inhibitory molecules, including the programmed cell death 1 protein (PD‐1), as well as T‐cell immunoglobulin mucin family member 3 (TIM‐3), lymphocyte‐activation gene 3 (LAG‐3), cytotoxic T‐lymphocyte antigen 4 (CTLA4), and 2B4, and also by the presence of excessive immunosuppressive signals within the liver microenvironment.( 16, 17, 18 ) Among these molecules, PD‐1 is the inhibitory receptor with the highest expression on HBV‐specific T cells within the liver, and the ligand of PD‐1 (programmed cell death 1 ligand 1 [PD‐L1]) is likewise increased on HBV‐infected hepatocytes.( 19, 20, 24 ) A high PD‐1 expression is therefore considered a classical hallmark of exhausted T cells (Fig. 1). In addition to inhibition of HBV‐specific T cells, HBV antigens can suppress the innate immune response and affect global and HBV‐specific B‐cell functions, which may further contribute to the status of immune exhaustion.( 21, 22, 23, 24 )
FIG. 1.
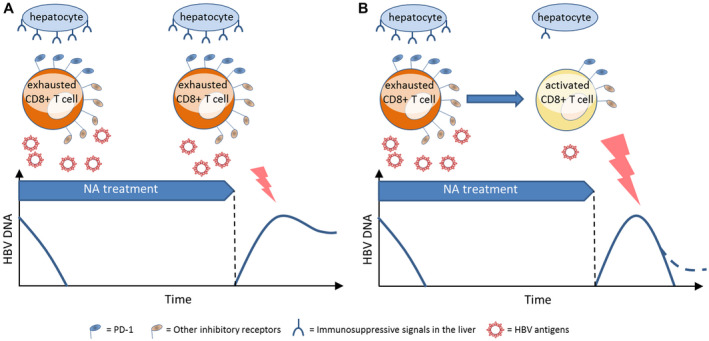
Immunological and viral factors that are believed to determine response to NA treatment discontinuation. (A) CD8+ T cells that are chronically overstimulated by viral antigens (e.g., HBV DNA, HBsAg) and inhibited by excessive immunosuppressive microenvironment in the liver (e.g., PD‐L1) express high levels of inhibitory receptors, such as PD‐1, TIM‐3, LAG‐3, and CTLA‐4. The rebound of HBV replication after treatment discontinuation results in a relapse of high levels of HBV‐DNA replication in those patients who cannot establish sufficient immune control due to persisting T‐cell exhaustion. (B) Patients who overcome T‐cell exhaustion during NA treatment may respond to the HBV‐DNA rebound by establishing immune control with low or undetectable levels of HBV DNA, ideally followed by HBsAg loss. Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte antigen 4; LAG‐3, lymphocyte‐activation gene 3; TIM‐3, T‐cell immunoglobulin mucin family member 3.
Continuous research focuses on whether immune exhaustion in chronic hepatitis B can be overcome by long‐term NA treatment and the consecutive reduction of HBV antigens, primarily HBV DNA.( 11 ) Indeed, a woodchuck model of HBV infection showed that entecavir (ETV) treatment significantly decreased PD‐1 expression on CD8 T cells in chronic carriers.( 25 ) Consequently, the question arises as to whether a CD8 T‐cell PD‐1 expression that has decreased during NA treatment represents a more promising starting position for treatment discontinuation. A complementary small clinical study showed an association of the PD‐1 status of specific T‐cell populations for the viral flare following NA discontinuation.( 26 )
However, even during long‐term treatment, the number of patients achieving HBsAg loss remains stable. It seems doubtful that changing the immune environment alone is sufficient to enhance T cell–based immune control of HBV infections. Accordingly, a recent study in a murine model of HBV infections showed that exhausted CD8+ T cells undergo a stable form of dysfunctional differentiation during chronic HBV replication, but that switching immune environment alone could not reconstitute their antiviral function. During chronic HBV infection, PD‐L1 blockade alone showed no effect on restoring viral‐specific T‐cell response and controlling viral infection.( 25 ) However, it seems possible that a restored immune response against HBV will be the prerequisite for a de novo response against it during chronic infections (Fig. 1). The reappearance of HBV replication as frequently seen after stopping long‐term NA treatment and often considered as an unwanted virologic relapse could in fact be the desired trigger for this immune response, and the collateral biochemical flare appears to be its surrogate. This assumed effect of HBV‐DNA reappearance following NA discontinuation has also been designated as “auto‐vaccination.” The effect of the rebound in HBV‐DNA levels on the immune status was shown in a small prospective NA stop study, in which the PD‐1 expression on CD8+ T cells and several plasma cytokines, chemokines, and growth factors indicating T‐cell activity was shown to increase after the reappearance of HBV DNA.( 27, 28 )
Response to NA Discontinuation Runs Through Different Phases
The present studies suggest that in the period after NA discontinuation, patients run through different phases, all of which deserve special attention and measures by the treating physician to provide optimal care for the patient. Although there is no universal agreement on the categorization of these phases, we suggest a classification of four post‐NA treatment phases that have important clinical relevance and describe their individual characteristics (Fig. 2).
FIG. 2.
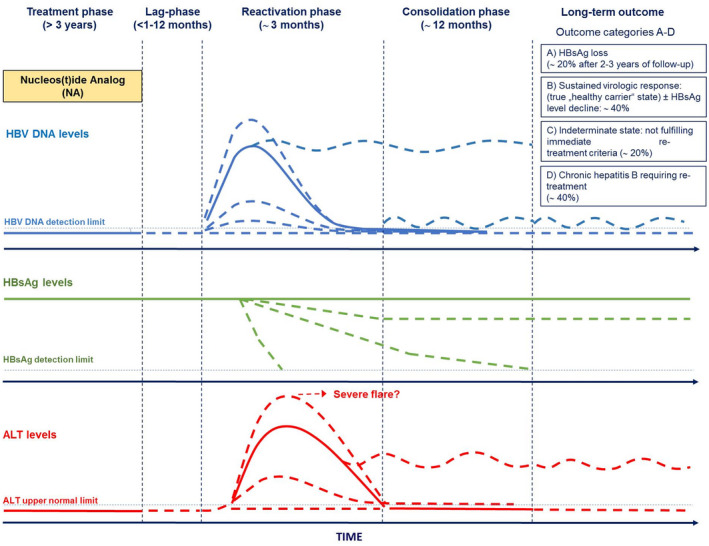
Proposed phases of hepatitis B reactivation after NA discontinuation and possible long‐term outcomes. (Adapted from Ref. 89.)
Lag Phase
The lag phase describes the period that lies between the point of NA discontinuation and the reappearance of HBV DNA. During the lag phase, alanine aminotransferase (ALT) levels and liver function are normal, and the patient should be free of complaints associated with NA discontinuation. Most viral relapses will likely occur shortly after NA discontinuation (i.e., within the first 3‐6 months); however, the duration until the occurrence of the relapse may vary, and relapses may even occur after more than 12 months.( 32, 33, 62, 68 ) All patients undergoing NA discontinuation need to be monitored for high rates of HBV‐DNA relapses, as described in many studies. Accordingly, in a prospective study in 184 HBeAg‐negative Asian patients, the relapse rate was 74.3% at month 12 after NA discontinuation, with a peak of relapses between months 3 and 6, and relapses increased to 91.4% at month 12 after NA discontinuation (Fig. 3).( 59 )
FIG. 3.
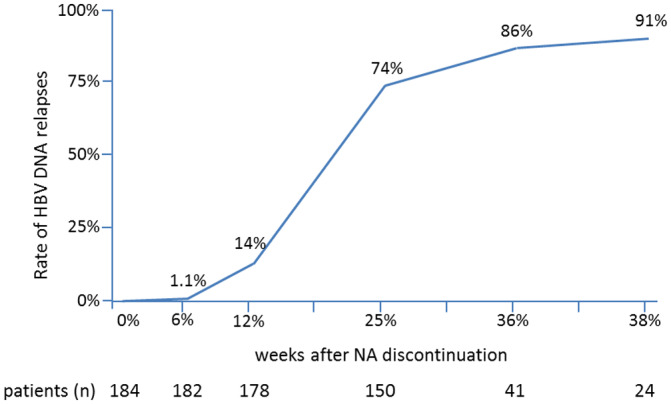
Increase of HBV‐DNA relapses greater than 2,000 IU/mL in HBeAg‐negative patients after discontinuing treatment with ETV. Most virologic relapses occurred between weeks 12 and 24. At 48 weeks after NA discontinuation, a virologic relapse had appeared in almost all patients. (Adapted from Fig. 1 of Ref. 59.)
Factors such as the duration of the lag phase are not well defined; however, it appears that HBsAg levels at the point of NA discontinuation may play a role. Some studies have shown that response is achieved more frequently and earlier in those patients with low HBsAg levels at the start of NA discontinuation.( 32, 33, 34, 49 ) Moreover, evidence from recent studies in Asian patients shows that viral relapses occur earlier after withdrawal of TDF than after withdrawal of ETV. Accordingly, in a recent prospective study from Taiwan, the median time to virologic relapse was significantly shorter in patients who had received TDF (n = 34) compared to those who had received ETV (n = 66; 2.4 vs. 6.2 months; P = 0.035).( 29 ) This trend is also evident in 220 Asian HBeAg‐negative patients from the prospective ABX203 vaccine study who underwent withdrawal of either ETV or TDF.( 30 ) Also in these patients, the type of antiviral therapy was significantly associated with early virological relapse, defined as an HBV‐DNA load of greater than 2,000 IU/mL until week 12, and relapse occurred earlier in patients treated with TDF versus those treated with ETV (median time, 6 versus 24 weeks; P < 0.001). Other factors that potentially influence the duration of the lag phase could be the consolidation treatment duration after HBV DNA has become undetectable, the immune status (including host genetic variants), and the HBV genotype.( 29, 32, 45, 49 )
Reactivation Phase
The reactivation phase starts with the reappearance of HBV DNA in patients undergoing NA discontinuation, which can be expected in 70%‐100% of patients (Fig. 3).( 32, 63, 70
) In many cases, this is followed by an ALT flare. The reactivation phase ends when HBV DNA and ALT levels return to a stable plateau (Fig. 2).
Consolidation Phase
The consolidation phase is the period after the reactivation of the HBV infection, in which the extent of achieved immune control following NA becomes apparent (Fig. 2).( 26, 28 ) The duration of this phase is undefined. It is therefore possible that HBsAg has already become undetectable during the reactivation phase, and the endpoint of NA discontinuation has already been reached. On the other hand, HBsAg losses can still occur years after the relapse of HBV‐DNA levels has occurred, which means that patients have to be followed up for a long time to fully assess the success of the intervention.
Long‐Term Outcome
The long‐term effect of NA discontinuation, including both the stability of HBsAg loss and the possible occurrence of further HBsAg losses, is unknown. Interestingly, some patients were shown to exhibit continuous decreases in HBsAg levels after NA withdrawal, and it needs to be shown in long‐term observational studies whether such patients will lose HBsAg in the long term.( 62 )
NA Discontinuation in HBeAg‐Negative Patients May Result in Beneficial HBsAg‐Positive Endpoints
In 2006, Hadziyannis et al. published a small uncontrolled single‐center study of the posttreatment course of 33 patients with HBeAg‐negative chronic hepatitis B who had discontinued adefovir dipivoxil after a 3‐5‐year treatment period.( 31 ) HBsAg clearance occurred during the long‐term follow‐up in 39% of patients at 69 months after NA withdrawal. Remarkably, 18 patients (55%) had achieved a sustained biochemical and virologic remission, a stage characterized by normal ALT levels and HBV‐DNA levels below the threshold for treatment indication (>2,000 IU/mL), which resembles the inactive chronic HBV infection (carrier) stage.( 32 ) Another endpoint described in this and other studies includes inducing a stage in which, although HBV replication increased to greater than 2,000 IU/mL but less than 20,000 IU/mL, there is an absence of signs of liver damage, with ALT values staying normal throughout the observation periods. We consider this stage an “indeterminate state,” and patients in this situation do not require immediate NA treatment according to current guidelines.( 6, 7, 8 )
One should consider that such HBsAg‐positive endpoints may be unsatisfactory or unstable, and the remaining long‐term risks are to date undefined. However, they likely represent stages in which no or only a very low disease progression needs to be anticipated. It seems reasonable that such patients should be monitored similarly to other patients with HBeAg‐negative chronic HBV infection.( 6, 7, 8 ) Thus, a close follow‐up with ALT and HBV‐DNA measurements every 3 months after the reactivation phase during the first year and at least every 6 months thereafter is recommended. If, after 3 years of follow‐up, patients still do not fulfill any indications for treatment but HBsAg remains detectable, lifelong monitoring like in all other patients in this phase of the infection is advisable.( 33 )
Evidence From Clinical Studies for the Effectiveness of NA Treatment Discontinuation
Following this observation, numerous, primarily nonrandomized studies, including heterogeneous patient populations assessing the response following NA treatment discontinuation, have been published.( 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 72 ) In most of these studies, discontinuing NA treatment in HBeAg‐negative patients led to a stable immune control in a number of patients, including functional cure, but also HBsAg‐positive stages without retreatment indication similar to the inactive carrier state. However, the great variability of the response rates makes it difficult to find a general consensus on the efficacy of this approach. Moreover, those studies did not follow uniform inclusion criteria or definitions of response or retreatment criteria, and only a few were conducted in a randomized controlled design.
A meta‐analysis in 1,716 patients from 25 of these studies, including 733 (42.7%) HBeAg‐positive and 967 (56.4%) HBeAg‐negative, primarily Asian patients, found a response to NA withdrawal after 12, 24, and 36 months in 51.4%, 39.3%, and 38.2% of patients, respectively.( 71 ) Although a response to NA withdrawal was defined differently in many of these studies, it always included low HBV‐DNA levels (<2,000 IU/mL in most studies) and normal or close to normal ALT levels. The overall pooled rate of a durable response in HBeAg‐negative patients was 38.0%. Of the patients who had achieved HBeAg loss during NA treatment, 88% maintained undetectable HBeAg for 36 months after NA withdrawal. HBsAg loss was found in a mean of 2% of patients.
Very few studies were conducted prospectively (Table 1).( 26, 41, 45, 59, 60, 61, 62, 68, 70, 72 ) Their results, however, differ strongly regarding sustained immune control and HBsAg loss rates, which ranged from 0%‐25% of patients. Up to 74% of patients did not require retreatment at the end of observation. Patient selection may be a reason for those differences. For example, in one recent prospective trial conducted in Asian patients, 1 of 27 (4%) HBeAg‐negative patients who stopped NA treatment achieved HBsAg loss after 72 weeks.( 68 ) In contrast, in another prospective trial that randomized 42 HBeAg‐negative, primarily Caucasian patients to stop or continue NA therapy, 19% of those patients who stopped therapy achieved HBsAg loss by week 144 off therapy (6.3% annually).( 62 ) Although at first sight both studies appear to include similar patient cohorts, the patient populations showed differences, for example, in the duration of previous NA treatment, which could be shorter in the first study, and HBeAg status at the start of NA treatment. In the first study, patients who also achieved HBeAg loss during NA treatment could be included, whereas in the second study, patients had to be HBeAg‐negative at NA initiation. Indeed, regarding the higher level of HBV replication and functionally impaired immune cells (e.g., natural killer and T cells), it seems questionable whether HBeAg‐positive patients who lose HBeAg during NA treatment have the same probability to respond to NA withdrawal as patients entering NA treatment in the HBeAg‐negative stage.( 73 ) However, investigating such differences has to date not been addressed in clinical studies. In addition to HBeAg status, different durations in observation periods across the studies after NA discontinuation may play a role in the different response rates. In one cohort that was prospectively observed over 144 weeks, HBsAg seroconversion rates doubled between weeks 72 and 144 after NA discontinuation; therefore, a further increase cannot be excluded (Fig. 4).
TABLE 1.
Selected Prospective Studies Assessing NA Discontinuation in HBeAg‐Negative Patients
| Study | Region | Design | HBeAg‐Negative Patients With NA Discontinued (n) | Endpoint(s) | NA | Mean NA Treatment Duration, Range (Months) | Mean Post‐NA Follow‐up, Range (Months) | HBV‐DNA Relapses (%) | HBsAg Loss (%) | No Retreatment (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Liu et al.( 41 ) | China | Prospective | 61 | HBV‐DNA relapse; HBsAg loss | LMV +/− PEG‐IFN | 30 (24‐66) | 22 (1‐84) | 56 | 10 | N/A |
| Ha et al.( 45 ) | China | Prospective | 145 | HBV‐DNA relapse; HBsAg loss | ADV +/− IFN‐α | 26 (24‐66)* | 16 (1‐88)* | 65.5 | 8.3 | N/A |
| Seto et al.( 59 ) | China | Prospective | 184 | HBV‐DNA relapse; HBsAg loss; immune control | ETV | ≥24 | 12 | 91.4 | 0 | 8.4† |
| Karakaya et al.( 60 ) | Turkey | Prospective | 23 | HBV‐DNA relapse; HBsAg loss | LMV | >60 | 12‐60 | 45 | 9 | 9 |
| Cao et al.( 61 ) | China | Prospective | 22 | HBV‐DNA relapse; HBsAg loss | ETV, ADV, LdT, LMV | 47 (29‐77) | N/A | 53 | N/A | N/A |
| Berg et al.( 62 ) | Germany | Prospective, randomized | 21 | HBsAg loss; retreatment | TDF | 33 | 36 | 100 | 19 | 43 |
| Rivino et al.( 26 ) | Great Britain | Prospective | 21/27 | HBV‐DNA relapse | TDF, LMV | ≥24/≥24 | 12 | N/A | 0 | 19/51 |
| Papatheodoridis et al.( 63 ) | Greece | Prospective | 57 | HBV‐DNA relapse; retreatment | ETV, TDF | >48 | 18 | 72 | 25 | 74† |
| Liem et al.( 68 ) | Asian patients (98%) | Prospective, randomized | 27 | HBV DNA < 2,000 IU/mL; HBsAg loss | TDF, ETV | 91 | 18 | 100 | 1 (2.2) | 17 (38%) |
| van Bömmel et al.( 70 ) | European patients | Prospective, randomized | 79 | HBsAg loss | TDF, ETV, LdT, LMV | >48 | 24 | 100 | 8 (10.3) | 53 (67.9%) |
Any ALT increase.
Any HBV‐DNA increase.
Abbreviations: ADV, adefovir dipivoxil; IFN, interferon; N/A, not applicable; PEG‐IFN, pegylated interferon.
FIG. 4.
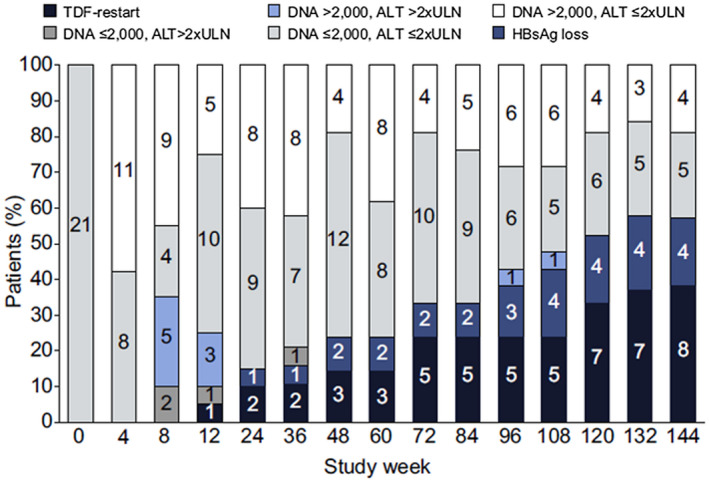
Development of different forms of response during the 14 weeks after prospective discontinuation of TDF in the FINITE trial.( 62 ) The numbers of patients in different stages of HBV replication and ALT activity are shown. Despite a relapse in HBV replication in all of the patients and ALT flares in some of the patients, most patients entered an HBsAg‐positive chronic infection (carrier) phase (white, gray) or achieved an HBsAg loss (dark blue) during the course of the study. One third of the patients required retreatment (black).
Few studies have included a randomized control group.( 62, 68, 70 ) The Stop NUC study, presented at the European Association for the Study of the Liver’s Digital International Liver Congress in 2020, is the first prospective randomized study to fully recruit a predefined number of patients. In this study, 166 patients without cirrhosis but with HBeAg‐negative hepatitis B were randomized into a NA stop arm and an NA continuation arm and were followed for 96 weeks. At the end of the observation period, 8 patients in the stop arm (10.3%) and no patient in the discontinuation arm had achieved HBsAg loss. In the stop arm, 67.9% of patients had no immediate indication for NA therapy according to current guidelines.( 70 )
Factors Associated With Response to NA Discontinuation
Patient Origin and HBV Genotype
Response to NA discontinuation was reported in patients from Asia as well as in European patients (Table 1). The HBV genotype (primarily genotypes B and C) was described in some studies; however, it was not associated with a response to NA discontinuation. Those results need to be interpreted cautiously, because the total numbers of patients with different genotypes are small, and there is no direct comparison. It is widely accepted that when treating chronic hepatitis B, the origin of the patients has a strong impact on the serologic response; therefore its role in response to NA discontinuation obviously needs to be investigated in prospective and randomized trials.( 6 )
Duration and Type of NA Treatment
The extent to which NA treatment duration before discontinuation affects the likelihood of achieving a sustained response with or without HBsAg loss is still not well defined. However, it is widely agreed that NA discontinuation should not be considered in HBeAg‐negative patient populations after only very short treatment durations. In a meta‐analysis, a duration of an on‐treatment suppression of HBV DNA to undetectable levels less than 24 months before stopping NAs was associated with higher retreatment rate as a treatment duration longer than 24 months (75% vs. 35.6%; P = 0.005).( 71 ) The highest response rates to NA discontinuation were reported in European studies. Accordingly, in Greek HBeAg‐negative patients with virologic remission for a mean of 5.3 years, HBsAg loss occurred in 25% of patients.( 63 ) In two prospective cohorts from Germany with virologic response to NAs for more than 4 years, the HBsAg loss rates following NA discontinuation were 19% and 10%, respectively.( 62, 70 ) However, it is unclear whether the apparently lower response rates in Asian studies are due to the shorter treatment duration or possibly to ethnic factors or the HBV genotype. In conclusion, the optimal duration of virologic response before NA discontinuation remains unclear, and it is questionable whether it increases in a linear trajectory, with increasing treatment durations beyond year 4 to 5. The follow‐up analysis of 106 HBeAg‐negative patients who received TDF for 8 years in the randomized controlled study GS‐US‐174‐0102 is the only analysis to include a strictly defined, long‐term duration of NA treatment.( 67 ) Only a subset of patients could be followed until week 24 after treatment (63 of 106): 5% had HBsAg loss, and 35% had HBV‐DNA concentrations of less than 2,000 IU/mL and ALT concentrations less than the upper limit of normal (ULN).
The antiviral potency of the NA regimen may also influence the rate of off‐treatment response, and there are hints that more potent NA may lead to a higher rate of patients showing sustained immune control after NA discontinuation, as compared with those being treated with first‐generation NAs. In an Asian study of patients who were treated with either ETV, lamivudine (LMV), or telbivudine (LdT), the 1‐year cumulative relapse rate after cessation of ETV therapy was 45.3%, which was significantly lower, and relapses occurred later than those after cessation of LMV or LdT (P = 0.027).( 49 )
Timing of Retreatment
Immediate start of retreatment during the reactivation phase may negatively impact the chance of achieving a functional cure. This is also probably the most important lesson learned from the Hadziyannis study, which was the first study to evaluate long‐term outcome after controlled discontinuation of long‐term NA treatment. In this study, the investigators postponed retreatment as long as possible, although virologic relapse was universal. They observed the highest rate of HBsAg loss ever reported (39%).( 32 ) This concept is supported by more recent findings in which patients with clinical relapses who remained untreated had a significantly higher incidence of HBsAg clearance as compared with those who were retreated (19% and 1.7%, respectively) (Fig. 5).( 65 ) As long as there is no severe and potentially life‐threatening flare, patients should be followed until they have entered the consolidation phase.
FIG. 5.
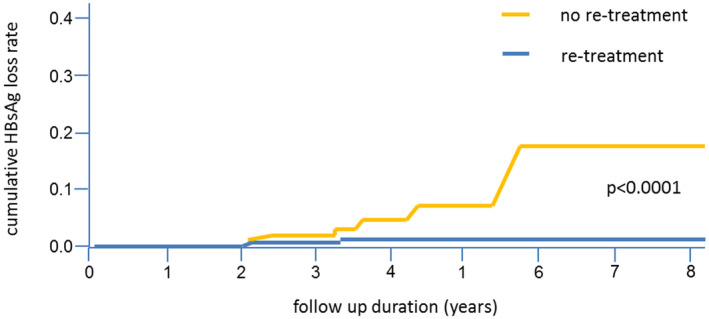
Cumulative HBsAg loss rate in 519 patients with clinical relapse and retreatment (blue line; n = 269) or patients with clinical relapse but no retreatment (yellow line; n = 150). (Adapted from Fig. 3 of Ref. 65.)
HBsAg Levels at NA Discontinuation
Although the level of HBsAg has been found to be a marker for post‐NA treatment response in many studies, results are contradictory. Chen et al. found that HBsAg cutoff values of 120 and 200 IU/mL were predictive of a response to treatment.( 64 ) Similarly, Lee et al. identified serum HBsAg levels of less than 2 log10 IU/mL to be a reliable predictor of a sustained off‐treatment response.( 56 ) In a large retrospective cohort of 691 HBeAg‐negative patients with chronic HBV infection who had discontinued NA treatment, during a median follow‐up period of 155 (2‐614) weeks after cessation of NA treatment, virologic relapse and clinical relapse were documented in 547 patients (79.2%) and 419 patients (60.6%), respectively.( 65 ) In this study, lower baseline HBsAg levels were the only statistically significant baseline factor (median, 2.7 vs. 3.1 log10 IU/mL; P = 0.0004) associated with HBsAg loss. In the Stop‐NUC study in primarily European patients, HBsAg loss rates were 28% in patients with HBsAg levels less than 1,000 U/mL at baseline as compared with only 1.9% in those patients with higher HBsAg levels.( 70 ) Other, more heterogenous studies showed a similar association with very low HBsAg levels (< 2 log10 IU/mL) and HBsAg loss after NA discontinuation.( 50, 60, 63 )
Response Prediction by Novel Serum Markers
New biomarkers have also been evaluated for their role in predicting response to NA discontinuation, although not yet in prospective studies. One of these markers is the hepatitis B core‐related antigen (HBcrAg), which is measured by an immunoassay that simultaneously detects HBeAg, hepatitis B core antigen, and a 22‐kDa truncated core‐related protein. Results from studies in Asian populations, primarily with HBV genotypes B and C infection, suggest that HBcrAg could be a potential marker for predicting a favorable outcome following discontinuation of NA therapy.( 74, 76 ) Low HBcrAg levels were associated with a lower risk of a biochemical relapse following NA discontinuation, and they were independent predictors of HBV‐DNA relapses.( 74, 76 ) Guidelines for using HBcrAg levels to estimate the risks following NA discontinuation have been proposed and incorporated in the Japan Society of Hepatology guidelines for the management of HBV infection.( 77 ) These recommendations are based on a score that includes HBsAg and HBcrAg levels for stopping NA therapy in patients who have been treated for at least 2 years with NAs for chronic hepatitis B and have undetectable serum HBV DNA and negative serum HBeAg.( 78 ) Thus, HBeAg‐negative patients who respond to NA treatment for at least 2 years and with both HBsAg levels less than 1.9 log10 IU/mL and HBcrAg levels less than 3.0 log10 IU/mL have a low risk of a virologic relapse after treatment withdrawal.
Serum HBV‐RNA levels during NA treatment as well as levels of intrahepatic HBV RNA have been suggested as another marker to detect a response to NA withdrawal.( 76, 78, 79, 80 ) Indeed, this marker provides information on covalently closed circular DNA transcriptional activity, even during the suppression of HBV DNA during NA treatment.( 80 ) Low‐serum HBV‐RNA levels might therefore be a useful predictor for durably low HBV replication after stopping NA treatment. However, the value of this marker has not yet been evaluated in a prospective trial. Also, there is no existing validated assay for serum HBV RNA, and the lower detection limit is undefined.
Apart from viral markers, markers for immune responsiveness are under investigation for their potential to predict a response to NA withdrawal. One focus of the research is to quantify T‐cell exhaustion by circulating or histological makers. However, currently there is no biomarker that can be used for patient selection for treatments with antibodies for inhibitory receptors such as PD‐1.( 81 ) Another approach is to monitor the innate immune response of circulating markers, such as the soluble growth stimulation expressed gene 2 (sST2), which is one of the toll‐like receptor (TLR)/interleukin‐1 receptor members involved in a variety of inflammatory and immune processes. In a study from China, sST2 with a small sample size was recently found to be increased in clinical relapse patients at week 12 after NA withdrawal.( 69, 73 ) However, large‐scale observations of the prediction of this marker are missing.
ALT Flares After NA Discontinuation Can Mostly Be Tolerated
Virological relapses can be observed in most patients with HBeAg‐negative chronic hepatitis B who discontinue NAs, and in many of those patients, biochemical relapses follow.( 32, 45, 49, 59, 62, 66, 70, 75 ) Actually, in patients without cirrhosis, flares with high ALT elevations (> 5‐10 times the ULN) appear seldom, and jaundices only exceptionally.( 61, 62, 63, 68, 70, 71 ) Although discontinuation of NA treatment is generally safe, hepatic decompensations and fatal courses following the chronic hepatitis B reactivation after NA discontinuation may occur (Table 2).( 82 ) Of note, the results of a recent meta‐analysis show that, to date, fatalities following the reactivation after NA discontinuation have only been observed in patients with preexisting liver cirrhosis.( 71 ) Alarmingly, the number of patients at risk in the studied population was high, with liver cirrhosis at baseline present in 243 of the 1,362 patients (17.8%) with reported signs of histological pathology. In the largest and most recent study, which included 308 patients with HBeAg‐negative chronic hepatitis B with compensated cirrhosis from Taiwan before treatment, and who had discontinued NA treatment according to the Asian Pacific Association for the Study of the Liver recommendations, the cumulative probability of liver decompensation was 1% over a follow‐up of more than 5 years, and 3 patients died.( 65 )
TABLE 2.
Relapses, Hepatic Decompensation, and Fatal Outcomes in HBeAg‐Negative Patients After NA Discontinuation (Selected Studies)
| Study | Number of Patients With Cirrhosis, n | HBV‐DNA Relapse, n (%) | ALT Relapse, n (%) | Hepatic Decompensation, n (% Cirrhosis) | Death, n (% Cirrhosis) |
|---|---|---|---|---|---|
| Lim et al.( 82 ) (two case reports) | 2 | 2 (100) | 2 (100) | 2 (100) | 1 (100) |
| Jeng et al.( 65 ) | 691 (308) | 547 (79.2)* | 419 (60.6)† | 9 (100) | 3 (100) |
| Kuo et al.( 66 ) | 22 | N/A | 82 (68.4) | 1 (100) | 1 (100) |
Increase of HBV DNA > 2,000 IU/mL.
increase of ALT > 2× ULN.
Because the relapse after NA discontinuation is not only associated with the risk of a severe flare but also a surrogate for the achievement of immune control, there is increasing interest in differentiating favorable from potentially unfavorable flares.( 32 ) It has therefore been suggested to interrupt ALT flares that do not appear simultaneously with a decrease in HBsAg.( 80 ) However, there is currently insufficient evidence that the extent and duration of the virologic and biochemical flares match to consecutive outcomes. Indeed, in a recent study in a population of 36 patients achieving sustained remission (HBV DNA <2000 IU/mL and normal ALT) after NA discontinuation, patients with or without ALT flares showed similar kinetics of HBsAg levels.( 84 )
Although data from large prospective and randomized studies are missing, retreatment with TDF has been shown to lead to a normalization of ALT and a decrease in HBV‐DNA levels in some patients with severe reactivation after discontinuation of TDF.( 62, 65 ) However, the study by Jeng et al. shows that early retreatment reduces the chance for HBsAg loss, which is why they should not be interrupted too early.( 65 )
Defining the nature of an ALT flare and the right time point for retreatment is not established, but is obviously crucial for the safety of the patient undergoing NA discontinuation as well as maximizing the efficacy of NA discontinuation. For this purpose, retreatment criteria have been recommended (Table 3).( 62 )
TABLE 3.
Proposed Retreatment Criteria for HBeAg‐Negative Patients After NA Discontinuation( 62 )
| NA treatment should be immediately re‐installed if one of the following criteria is met: |
| 1. Confirmed (i.e., two consecutive central laboratory results) increase in direct bilirubin from baseline and ALT ULN at the confirmatory test |
| 2. Confirmed sustained increase in prothrombin time ≥ 2.0 seconds from baseline with appropriate vitamin K levels and elevated ALT |
| 3. Confirmed elevated ALT 10× ULN with or without associated symptoms |
| 4. ALT 2× ULN and ≤ 5× ULN persisting for ≥ 84 days (12 weeks) as well as an HBV‐DNA relapse ≥ 20,000 copies/mL |
| 5. ALT 5× ULN and ≤ 10× ULN persisting for ≥ 28 days (4 weeks) |
Elevated HCC Risk After NA Discontinuation?
An increased risk of developing HCC due to a re‐increase of HBV replication after NA discontinuation may represent a drawback to this approach. Although the few data that have included HCC surveillance show no increase in HCC incidence related to the finite NA approach,( 49 ) careful HCC surveillance of patients who discontinue NA treatment needs to be maintained, especially if they have additional risk factors of developing HCC.
Consequences of NA Discontinuation Approach for Current New HBV Treatment Strategies
Direct‐acting antivirals under clinical investigation to treat chronic hepatitis B include entry inhibitors, core protein inhibitors, and RNA silencers. Immunotherapeutic approaches include TLR‐7 and TLR‐8 agonists, therapeutic vaccines, checkpoint inhibitors, retinoic acid inducible gene I (RIG‐I) agonist, and antibodies to HBV (anti‐HBV).( 85 ) Results of preclinical and early clinical studies are promising for higher functional cure rates as current treatments; however, long‐term rates of functional cure have not yet been shown. With respect to the available date on the efficacy of NA discontinuation, consideration of the use of such a treatment should be weighed against this approach, especially in patients with a high probability for a functional cure just by stopping NAs, and that may be, for example, identified by low HBsAg levels as well as by other markers that need to be defined.
Apart from representing a possible alternative, NA discontinuation might also be an enhancer of other approaches to induce functional cure in patients with chronic hepatitis B, especially because most patients eligible for those approaches will likely be under NA treatment. A major barrier to achieving a cure for chronic HBV infection by NA treatment is the presence of a dysfunctional immune response to the virus. Resolution of the T‐cell exhaustion is possible through blocking PD‐1, and this treatment approach was shown to improve specific anti‐HBV T‐cell responses in human intrahepatic T cells and mouse and woodchuck models.( 27, 86, 87 ) In this clinical study of nivolumab, a fully human immunoglobulin G4 in patients with advanced HCC (CheckMate 040 Study), 3 of 51 patients (6%) with chronic HBV infection who received 3.0 mg/kg every other week demonstrated a 1‐log decline in HBsAg.( 88 ) It might be possible that immune‐reinvigorating therapeutic approaches such as PD‐1 inhibition or a therapeutic vaccine may have a beneficial effect when applied to patients undergoing NA withdrawal. In addition, a combined boost of the immune system by different approaches might be effective to overcome immune exhaustion in HBV infections. In woodchucks with chronic woodchuck hepatitis virus infection, blocking PD‐L1 combined with DNA vaccination was effective at controlling HBV‐DNA levels, whereas NA treatment alone or in combination with a vaccine had no effect.( 87 )
A Practical Approach: When to Stop and How to Monitor
Discontinuation of NA may offer the chance for achieving a durable immune control of the infection in a selected but still significant number of patients with chronic HBeAg‐negative hepatitis B. This approach is generally safe; however, it should only be tried if certain prerequisites can be fulfilled by the patient as well as the treating physician (Fig. 6). Because this treatment approach is not well characterized yet, it is advisable to obtain written, informed consent from the patients. The physician conducting the NA discontinuation should be an expert in the field, and advanced fibrosis and liver cirrhosis need to be ruled out by a reliable method. A close follow‐up at least within the first year after NA discontinuation must be granted by both. For this, the recommendation is to monitor ALT levels at least on a monthly basis during the first months and until the consolidation phase has been entered, and on a biweekly basis when and as long as ALT has surpassed 5 times the ULN, to allow a timely reinstallation of NA treatment in case of severe ALT flares. The HBV‐DNA flares observed during the reactivation phase are often transient and most likely represent a trigger for inducing a long‐term immune control by specific CD8+ T cells, and therefore need close follow‐up evaluation but not immediate interventions. Harmful ALT flares need to be identified and can be interrupted by reintroducing NA treatment. Retreatment with NA can cut a relapse; however, it also lowers the likelihood of response (Fig. 7). Therefore, retreatment criteria have been proposed to allow certain virologic and biochemical relapses without putting patients in danger, and we recommend structuring and systematically including such guidelines if NA discontinuation is performed (Table 3).
FIG. 6.
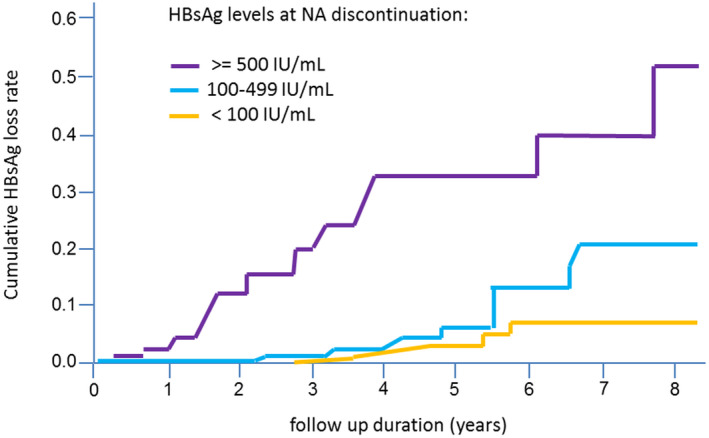
Cumulative HBsAg loss rates according to different HBsAg levels at the time point of NA treatment discontinuation (log‐rank test, P < 0.0001). (Adapted from Fig. 4 of Ref. 65).
FIG. 7.
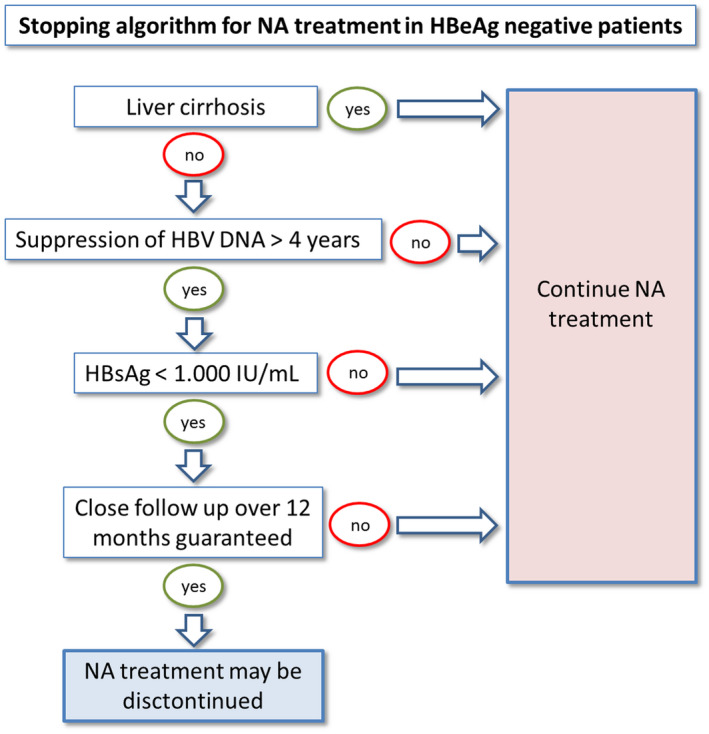
Suggested algorithm for planning NA discontinuation in HBeAg‐negative chronic hepatitis B. Of note, patients who show response to NA discontinuation should remain under surveillance for the development of hepatocellular carcinomas.
Conclusions
Currently, discontinuation of an effective long‐term NA treatment represents the most promising approach to achieve functional cure or treatment‐free survival for patients who are HBeAg‐negative. The probability of response is especially high in patients with low HBsAg levels (< 1,000 U/mL). The implementation of an NA interruption is expected to be further supported by treatment guidelines. However, for effective use of this approach, it will be necessary to better define the conditions for who is likely to respond and whether novel HBV biomarkers may be helpful in this context. It is important to distinguish different phases after NA discontinuation to understand the course of reactivation and provide adequate measures. Discontinuation of NA treatment should be restricted to patients without advanced fibrosis and to those for whom a close follow‐up can be guaranteed. The role of the HBV‐specific immune response at the time of NA cessation as well as the value of biomarkers to better predict the subsequent response to NA discontinuation need to be better understood to develop individualized treatment approaches. In addition, large‐scale studies are needed to confirm the retreatment guidelines we have proposed here. Finally, research is needed on how this treatment approach may add to the efficacy of novel treatment approaches, especially to those with an immune modulatory mechanism.
Acknowledgment
Open Access funding enabled and organized by Projekt DEAL.
Potential conflict of interest: Thomas Berg: Receipt of grants/research supports: Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Novartis, Sequana Medical, Receipt of honoraria or consultation fees: Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Roche, Sequana Medical, and Shionogi; Participation in a company sponsored speaker’s bureau: Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, and Sequana Medica. Florian van Bömmel: Receipt of grants/research supports: BMS, Gilead, Humedics, Roche, Janssen; Receipt of honoraria or consultation fees: Abbvie, Bayer, Gilead, Eisai, Ipsen, Janssen, MSD/Merck, Novartis, Roche; Participation in a company sponsored speaker’s bureau: Abbvie, Bayer, Gilead, Eisai, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, and Novartis.
conflict of interest: Dr. Berg consults for, advises, is on the speakers' bureau for, and received grants from Gilead, Janssen, MSD/Merck and AbbVie. Dr. van Bommel consults for, advises, is on the speakers' bureau for, and received grants from Gilead. He consults for, advises and received grants from Janssen and Roche.
View this article online at wileyonlinelibrary.com. DOI 10.1002/hep4.1708
References
- 1.World Health Organization . Hepatitis B. https://www.who.int/en/news‐room/fact‐sheets/detail/hepatitis‐b. Published July 27, 2020. Accessed December 2020. [Google Scholar]
- 2.Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology 2009;49(Suppl.):S35‐S44. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582‐592. [DOI] [PubMed] [Google Scholar]
- 4.Zoulim F. Inhibition of hepatitis B virus gene expression: a step towards functional cure. J Hepatol 2018;68:386‐388. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Gane EJ, Flisiak R, Trinh H, Petersen J, Gurel S, et al. Long term treatment with tenofovir disoproxil fumarate for chronic hepatitis B infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two phase 3 trials. Hepatology 2014;60:313A‐317A. [Google Scholar]
- 6.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, Lok ASF, McMahon BJ, Chang K‐M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009;58:974‐982. [DOI] [PubMed] [Google Scholar]
- 10.Zajac AJ, Blattman JN, Murali‐Krishna K, Sourdive DJD, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998;188:2205‐2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)‐specific T‐cell dysfunction in chronic HBV infection. J Virol 2007;81:4215‐4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PTF, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 2008;205:2111‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoletti A, Kennedy P. HBV antiviral immunity: not all CD8 T cells are born equal. Gut 2019;68:770‐773. [DOI] [PubMed] [Google Scholar]
- 14.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012;61(Suppl. 1):i6‐i17. [DOI] [PubMed] [Google Scholar]
- 15.Thimme R, Dandri M. Dissecting the divergent effects of interferon‐alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol 2013;58:205‐209. [DOI] [PubMed] [Google Scholar]
- 16.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim‐3/galectin‐9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One 2012;7:e47648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen‐4 on apoptosis‐prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011;53:1494‐1503. [DOI] [PubMed] [Google Scholar]
- 19.Chen JI, Wang X‐M, Wu X‐J, Wang Y, Zhao H, Shen B, et al. Intrahepatic levels of PD‐1/PD‐L correlate with liver inflammation in chronic hepatitis B. Inflamm Res 2011;60:47‐53. [DOI] [PubMed] [Google Scholar]
- 20.Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T‐cell responses can be restored by blocking programmed death‐1 pathway in chronic hepatitis B. Gastroenterology 2010;138:682‐693, e681‐684. [DOI] [PubMed] [Google Scholar]
- 21.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, et al. Regulation of toll‐like receptor‐2 expression in chronic hepatitis B by the precore protein. Hepatology 2007;45:102‐110. [DOI] [PubMed] [Google Scholar]
- 22.Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HLA, van der Molen RG, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology 2009;126:280‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll‐like receptor signaling pathway. J Hepatol 2011;55:762‐769. [DOI] [PubMed] [Google Scholar]
- 24.Salimzadeh L, Le Bert N, Dutertre CA, Gill US, Newell EW, Frey C, et al. PD‐1 blockade partially recovers dysfunctional virus‐specific B cells in chronic hepatitis B infection. J Clin Invest 2018;128:4573‐4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus‐specific immunity in vivo by combining therapeutic vaccination and PD‐L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014;10:e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, et al. Hepatitis B virus‐specific T cells associate with viral control upon nucleos(t)ide‐analogue therapy discontinuation. J Clin Invest 2018;128:668‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinker F, Zimmer CL, Höner Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, et al. Hepatitis B virus‐specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg‐negative chronic hepatitis B. J Hepatol 2018;69:584‐593. [DOI] [PubMed] [Google Scholar]
- 28.Höner Zu Siederdissen C, Rinker F, Maasoumy B, Wiegand SB, Filmann N, Falk CS, et al. Viral and host responses after stopping long‐term nucleos(t)ide analogue therapy in HBeAg‐negative chronic hepatitis B. J Infect Dis 2016;214:1492‐1497. [DOI] [PubMed] [Google Scholar]
- 29.Su TH, Yang HC, Tseng TC, Liou JM, Liu CH, Chen CL, et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis 2018;217:1193‐1201. [DOI] [PubMed] [Google Scholar]
- 30.Höner zu Siederdissen C, Hui AJ, Sukeepaisarnjaroen W, Tangkijvanich P, Su WW, Nieto GEG, et al. Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen‐negative patients. J Infect Dis 2018;218:1480‐1484. [DOI] [PubMed] [Google Scholar]
- 31.Hadziyannis SJ, Seveastianos V, Rapti IN, Vassilopoulos D, Hadziyannis E. Sustained biochemical and virological remission after discontinuation of 4 to 5 years of adefovir dipivoxil (ADV) treatment in HBeAg‐negative chronic hepatitis B [Abstract]. Hepatology 2006;44(Suppl. 1):231A. [Google Scholar]
- 32.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg‐negative patients with chronic hepatitis B who stop long‐term treatment with adefovir. Gastroenterology 2012;143:629‐636. [DOI] [PubMed] [Google Scholar]
- 33.Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow‐up and indications for liver biopsy in HBeAg‐negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol 2012;57:196‐202. [DOI] [PubMed] [Google Scholar]
- 34.Fung SK, Wong F, Hussain M, Lok ASF. Sustained response after a 2‐year course of lamivudine treatment of hepatitis B e antigen–negative chronic hepatitis B. J Viral Hepat 2004;11:432‐438. [DOI] [PubMed] [Google Scholar]
- 35.Enomoto M, Tamori A, Kohmoto MT, Hayashi T, Morikawa H, Jomura H, et al. Optimal duration of additional therapy after biochemical and virological responses to lamivudine in patients with HBeAg‐negative c hepatitis B: a randomized trial. Hepatol Res 2008;38:954‐959. [DOI] [PubMed] [Google Scholar]
- 36.Yeh CT, Hsu CW, Chen YC, Liaw YF. Withdrawal of lamivudine in HBeAg‐positive chronic hepatitis B patients after achieving effective maintained virological suppression. J Clin Virol 2009;45:114‐118. [DOI] [PubMed] [Google Scholar]
- 37.Fung J, Lai C‐L, Tanaka Y, Mizokami M, Yuen J, Wong D‐H, et al. The duration of lamivudine therapy for chronic hepatitis B: cessation vs. continuation of treatment after HBeAg seroconversion. Am J Gastroenterol 2009;104:1940‐1946. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Liu F, Liu Y‐D, Li X‐Y, Wang J‐B, Zhang Z‐H, et al. Stringent cessation criterion results in better durability of lamivudine treatment: a prospective clinical study in hepatitis B e antigen–positive chronic hepatitis B patients. J Viral Hepat 2010;17:298‐304. [DOI] [PubMed] [Google Scholar]
- 39.Kuo Y‐H, Chen C‐H, Wang J‐H, Hung C‐H, Tseng P‐L, Lu S‐N, et al. Extended lamivudine consolidation therapy in hepatitis B e antigen–positive chronic hepatitis B patients improves sustained hepatitis B e antigen seroconversion. Scand J Gastroenterol 2010;45:75‐81. [DOI] [PubMed] [Google Scholar]
- 40.Cai W, Xie Q, An B, Wang H, Zhou X, Zhao G, et al. On‐treatment serum HBsAg level is predictive of sustained off‐treatment virologic response to telbivudine in HBeAg‐positive chronic hepatitis B patients. J Clin Virol 2010;48:22‐26. [DOI] [PubMed] [Google Scholar]
- 41.Liu F, Wang L, Li XY, Liu YD, Wang JB, Zhang ZH, et al. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen–negative chronic hepatitis B patients. J Gastroenterol Hepatol 2011;26:456‐460. [DOI] [PubMed] [Google Scholar]
- 42.Jung YK, Yeon JE, Lee KG, Jung ES, Kim JH, Kim JH, et al. Virologic response is not durable after adefovir discontinuation in lamivudine‐resistant chronic hepatitis B patients. Korean J Hepatol 2011;17:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off‐treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen–negative patients. Antivir Ther 2011;16:1249‐1257. [DOI] [PubMed] [Google Scholar]
- 44.Chaung KT, Ha NB, Trinh HN, Garcia RT, Nguyen HA, Nguyen KK, et al. High frequency of recurrent viremia after hepatitis B e antigen seroconversion and consolidation therapy. J Clin Gastroenterol 2012;46:865‐870. [DOI] [PubMed] [Google Scholar]
- 45.Ha M, Zhang G, Diao S, Lin M, Sun L, She H, et al. A prospective clinical study in hepatitis B e antigen–negative chronic hepatitis B patients with stringent cessation criteria for adefovir. Arch Virol 2012;157:285‐290. [DOI] [PubMed] [Google Scholar]
- 46.Song MJ, Song DS, Kim HY, Yoo SH, Bae SH, Choi JY, et al. Durability of viral response after off‐treatment in HBeAg positive chronic hepatitis B. World J Gastroenterol 2012;18:6277‐6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He D, Guo S, Chen W, Chen X, Yan G, Wang J, et al. Long‐term outcomes after nucleos(t)ide analogues discontinuation in chronic hepatitis B patients with HBeAg‐negative. BMC Infect Dis 2013;13:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YJ, Kim K, Hwang SH, Kim SS, Lee D, Cheong JY, et al. Durability after discontinuation of nucleos(t)ide therapy in chronic HBeAg negative hepatitis patients. Clin Mol Hepatol 2013;19:300‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeng W‐J, Sheen I‐S, Chen Y‐C, Hsu C‐W, Chien R‐N, Chu C‐M, et al. Off‐therapy durability of response to entecavir therapy in hepatitis B e antigen–negative chronic hepatitis B patients. Hepatology 2013;58:1888‐1896. [DOI] [PubMed] [Google Scholar]
- 50.Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, et al. Predictors of relapse in chronic hepatitis B after discontinuation of anti‐viral therapy. Aliment Pharmacol Ther 2011;34:344‐352. [DOI] [PubMed] [Google Scholar]
- 51.Kwon JH, Jang JW, Choi JY, Park C‐H, Yoo SH, Bae SH, et al. Should lamivudine monotherapy be stopped or continued in patients infected with hepatitis B with favorable responses after more than 5 years of treatment? J Med Virol 2013;85:34‐42. [DOI] [PubMed] [Google Scholar]
- 52.Ridruejo E, Marciano S, Galdame O, Reggiardo MV, Muñoz AE, Adrover R, et al. Relapse rates in chronic hepatitis B naïve patients after discontinuation of antiviral therapy with entecavir. J Viral Hepat 2014;21:590‐596. [DOI] [PubMed] [Google Scholar]
- 53.Sohn HR, Min BY, Song JC, Seong MH, Lee SS, Jang ES, et al. Off‐treatment virologic relapse and outcomes of retreatment in chronic hepatitis B patients who achieved complete viral suppression with oral nucleos(t)ide analogs. BMC Infect Dis 2014;14:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patwardhan VR, Sengupta N, Bonder A, Lau D, Afdhal NH. Treatment cessation in noncirrhotic, e‐antigen negative chronic hepatitis B is safe and effective following prolonged anti‐viral suppression with nucleosides/nucleotides. Aliment Pharmacol Ther 2014;40:804‐810. [DOI] [PubMed] [Google Scholar]
- 55.He D, Guo S, Zhu P, Tao S, Li M, Huang H, et al. Long‐term outcomes after nucleos(t)ide analogue discontinuation in HBeAg‐positive chronic hepatitis B patients. Clin Microbiol Infect 2014;20:O687‐O693. [DOI] [PubMed] [Google Scholar]
- 56.Lee HA, Seo YS, Park SW, Park SJ, Kim TH, Suh SJ, et al. Hepatitis B surface antigen titer is a good indicator of durable viral response after entecavir off‐treatment for chronic hepatitis B. Clin Mol Hepatol 2016;22:382‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C‐H, Lu S‐N, Hung C‐H, Wang J‐H, Hu T‐H, Changchien C‐S, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol 2014;61:515‐522. [DOI] [PubMed] [Google Scholar]
- 58.Jiang J‐N, Huang Z‐L, He L‐X, Huang Y‐H, Su M‐H, Xie R, et al. Residual amount of HBV DNA in serum is related to relapse in chronic hepatitis B patients after cessation of nucleos(t)ide analogs. J Clin Gastroenterol 2014;49:323‐328. [DOI] [PubMed] [Google Scholar]
- 59.Seto W‐K, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut 2014;64:667‐672. [DOI] [PubMed] [Google Scholar]
- 60.Karakaya F, Özer S, Kalkan Ç, Tüzün EA, Çalışkan A, Keskin O, et al. Discontinuation of lamivudine treatment in HBeAg‐negative chronic hepatitis B: a pilot study with long‐term follow‐up. Antivir Ther 2017;22:559‐570. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Chi H, Yu T, Li Z, Hansen BE, Zhang X, et al. Off‐treatment hepatitis B virus (HBV) DNA levels and the prediction of relapse after discontinuation of nucleos(t)ide analogue therapy in patients with chronic hepatitis B: a prospective Stop study. J Infect Dis 2017;215:581‐589. [DOI] [PubMed] [Google Scholar]
- 62.Berg T, Simon K‐G, Mauss S, Schott E, Heyne R, Klass DM, et al. Long‐term response after stopping tenofovir disoproxil fumarate in non‐cirrhotic HBeAg‐negative patients: FINITE study. J Hepatol 2017;67:918‐924. [DOI] [PubMed] [Google Scholar]
- 63.Papatheodoridis GV, Rigopoulou E, Papatheodoriri M, Zachou K, Xourafas V, Gatselis NK, et al. DARING‐B: discontinuation of effective entecavir (ETV) or tenofovir (TDF) therapy in non‐cirrhotic HBeAg‐negative chronic hepatitis B (CHBe−) patients: final results of a prospective Greek study. Antivir Ther 2018;23:677‐685 [DOI] [PubMed] [Google Scholar]
- 64.Chen CH, Hung CH, Wang JH, Lu SN, Hu TH, Lee CM. Long‐term incidence and predictors of hepatitis B surface antigen loss after discontinuing nucleoside analogues in noncirrhotic chronic hepatitis B patients. Clin Microbiol Infect 2018;24:997‐1003. [DOI] [PubMed] [Google Scholar]
- 65.Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen‐negative chronic hepatitis B. Hepatology 2018;68:425‐434. [DOI] [PubMed] [Google Scholar]
- 66.Kuo M‐T, Hu T‐H, Hung C‐H, Wang J‐H, Lu S‐N, Tsai K‐L, et al. Hepatitis B virus relapse rates in chronic hepatitis B patients who discontinue either entecavir or tenofovir. Aliment Pharmacol Ther 2019;49:218‐228. [DOI] [PubMed] [Google Scholar]
- 67.Buti M, Wong DK, Gane E, Flisiak R, Manns M, Kaita K, et al. Safety and efficacy of stopping tenofovir disoproxil fumarate in patients with chronic hepatitis B following at least 8 years of therapy: a prespecified follow‐up analysis of two randomised trials. Lancet Gastroenterol Hepatol 2019;4:296‐304. [DOI] [PubMed] [Google Scholar]
- 68.Liem KS, Fung S, Wong DK, Yim C, Noureldin S, Chen J, et al. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut 2019;68:2206‐2213. [DOI] [PubMed] [Google Scholar]
- 69.Xie L, Liao G, Chen H, Xia M, Huang X, Fan R, et al. Elevated expression of serum soluble ST2 in clinical relapse after stopping long‐term nucleos(t)ide analogue therapy for chronic hepatitis B. BMC Infect Dis 2019;19:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Bömmel F, Stein K, Heyne R, Möller H, Petersen J, Buggisch P, et al. Response to discontinuation of long‐term nucleos(t)ide analogue treatment in HBeAg negative patients: results of the Stop‐NUC trial. J Hepatol 2020;73(Suppl. 1):S118‐S119. [Google Scholar]
- 71.Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology 2016;63:1481‐1492. [DOI] [PubMed] [Google Scholar]
- 72.Papatheodoridis GV, Rigopoulou EI, Papatheodoridi M, Zachou K, Xourafas V, Gatselis N, et al. DARING‐B: discontinuation of effective entecavir or tenofovir disoproxil fumarate long‐term therapy before HBsAg loss in non‐cirrhotic HBeAg‐negative chronic hepatitis B. Antivir Ther. 2018;23:677–685. [DOI] [PubMed] [Google Scholar]
- 73.Wang W‐T, Zhao X‐Q, Li G‐P, Chen Y‐Z, Wang L, Han M‐F, et al. Immune response pattern varies with the natural history of chronic hepatitis B. World J Gastroenterol 2019;25:1950‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu Y‐C, Nguyen MH, Mo L‐R, Wu M‐S, Yang T‐H, Chen C‐C, et al. Combining hepatitis B core‐related and surface antigens at end of nucleos(t)ide analogue treatment to predict off‐therapy relapse risk. Aliment Pharmacol Ther 2019;49:107‐115. [DOI] [PubMed] [Google Scholar]
- 75.Jung KS, Park JY, Chon YE, Kim H‐S, Kang W, Kim BK, et al. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol 2016;51:830‐839. [DOI] [PubMed] [Google Scholar]
- 76.Carey I, Gersch J, Wang BO, Moigboi C, Kuhns M, Cloherty G, et al. Pregenomic HBV RNA and hepatitis B core‐related antigen predict outcomes in hepatitis B e antigen‐negative chronic hepatitis B patients suppressed on nucleos(t)ide analogue therapy. Hepatology 2020;72:42‐57. [DOI] [PubMed] [Google Scholar]
- 77.Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology . JSH guidelines for the management of hepatitis B virus infection. Hepatol Res 2014;44(Suppl. S1):1‐58. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka E, Matsumoto A. Guidelines for avoiding risks resulting from discontinuation of nucleoside/nucleotide analogs in patients with chronic hepatitis B. Hepatol Res 2014;44:1‐8. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 2016;65:700‐710. [DOI] [PubMed] [Google Scholar]
- 80.Fan R, Zhou B, Xu M, Tan D, Niu J, Wang H, et al.; Chronic Hepatitis B Study Consortium . Association between negative results from tests for HBV DNA and RNA and durability of response after discontinuation of nucleos(t)ide analogue therapy. Clin Gastroenterol Hepatol 2020;18:719‐727.e7. [DOI] [PubMed] [Google Scholar]
- 81.Callahan MK, Postow MA, Wolchok JD. CTLA‐4 and PD‐1 pathway blockade: combinations in the clinic. Front Oncol 2014;4:e00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim SG, Wai CT, Rajnakova A, Kajiji T, Guan R. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut 2002;51:597‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liaw YF, Jeng WJ, Chang ML. HBsAg kinetics in retreatment decision for off‐therapy hepatitis B flare in HBeAg‐negative patients. Gastroenterology 2018;154:2280‐2281. [DOI] [PubMed] [Google Scholar]
- 84.Jeng WJ, Chang ML, Liaw YF. Off‐therapy precipitous HBsAg decline predicts HBsAg loss after finite entecavir therapy in HBeAg‐negative patients. J Viral Hepat 2019;26:1019‐1026. [DOI] [PubMed] [Google Scholar]
- 85.Lopatin U. Drugs in the pipeline for HBV. Clin Liver Dis 2019;23:535‐555. [DOI] [PubMed] [Google Scholar]
- 86.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD‐1:PD‐L1 interactions contribute to the functional suppression of virus‐specific CD8+ T lymphocytes in the liver. J Immunol 2007;178:2714‐2720. [DOI] [PubMed] [Google Scholar]
- 87.Zhang E, Zhang X, Liu J, Wang B, Tian Y, Kosinska AD, et al. The expression of PD‐1 ligands and their involvement in regulation of T cell functions in acute and chronic woodchuck hepatitis virus infection. PLoS One 2011;6:e26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El‐Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lampertico P, Berg T. Less can be more: A finite treatment approach for HBeAg‐negative chronic hepatitis B. Hepatology 2018. Aug;68:397–400. [DOI] [PubMed] [Google Scholar]


