Figure 3. Headache intensity, adverse events and plasma measurements.
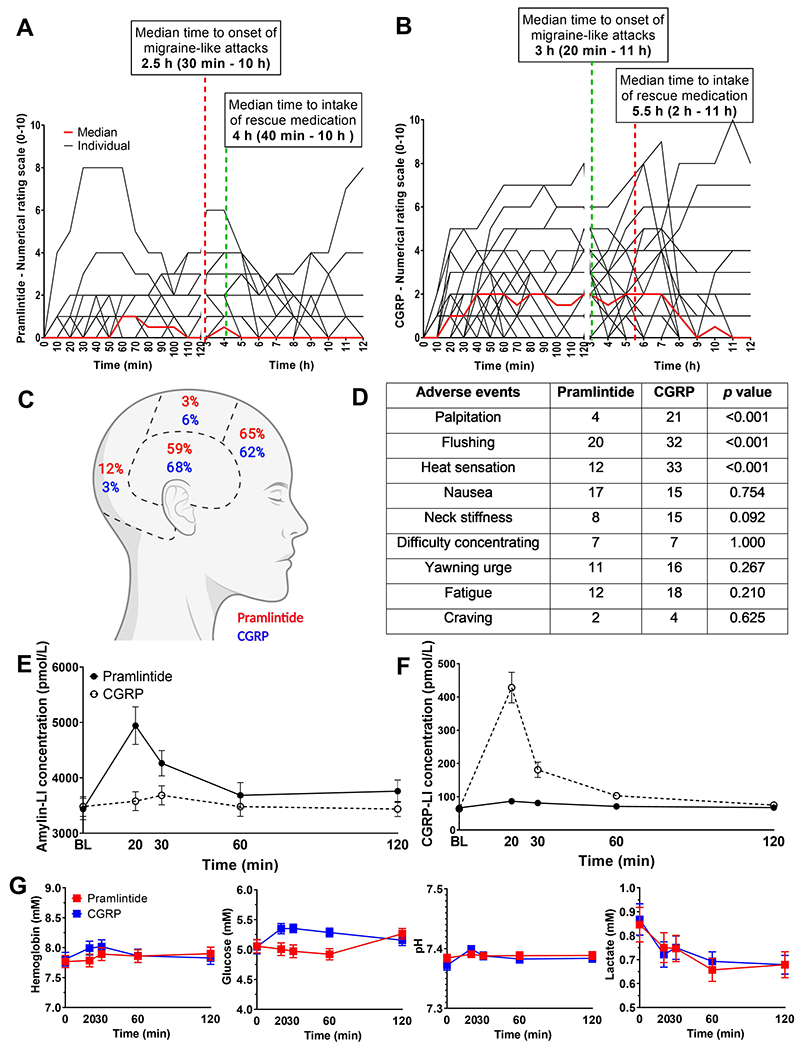
A) and B) Headache intensity in response to pramlintide or CGRP, respectively. Individual (black lines) and median (red line). C) Headache localization after pramlintide and CGRP. D) Incidence of adverse events (0-12 h) after CGRP or pramlintide, with statistical comparison between treatments E) and F) amylin-LI and CGRP-LI, respectively, in plasma samples from patients infused with pramlintide or CGRP, respectively. G) The concentration of hemoglobin, glucose, lactate and measurement of pH. Data are presented as mean ± SEM, except the headache intensity scores which are presented with individual data sets and median score. No statistical differences were found in incidence of headache and migraine-like attacks after pramlintide infusion compared to CGRP p = 0.375, p = 0.180.
