Figure 4.
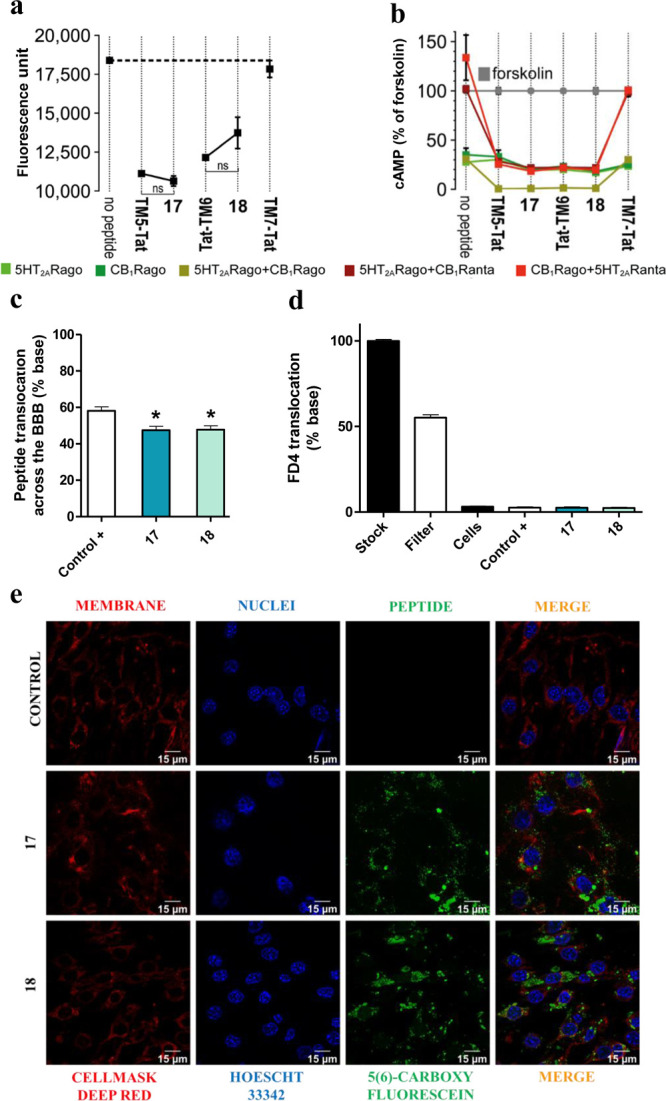
(a) BiFC analysis of the effect of peptides 17 and 18 on CB1R–5HT2AR heteromerization. Fluorescence (530 nm) of HEK-293T cells transfected with 5HT2AR-cYFP and CB1R-nYFP treated with a vehicle or peptide. Values are mean ± SEM of n = 10–30. TM5-Tat and Tat-TM6 are positive controls, whereas TM7-Tat is a negative control; ns (no significant), (one-way ANOVA followed by Tukey’s multiple comparison tests). (b) Effect of peptides 17 and 18 on cAMP production. Transfected cells preincubated with a vehicle (no peptide) or with 5HT2AR (5HT2ARago, DOI, 100 nM) or CB1R (CB1Rago, WIN, 100 nM) agonists or with 5HT2AR (5HT2ARanta, MDL, 300 nM) or CB1R (CB1Ranta, RIM, 1 μM) antagonists and combinations thereof, in the presence of FK. Values are mean ± SEM of n = 3–6 of FK-treated cells. Two-way ANOVA followed by Tukey’s multiple comparison tests was used to analyze the data (Table S5). (c) In vitro translocation of peptides 17 and 18. Translocation (%) across the transwell BBB model quantified as fluorescence in the basolateral chamber after 24 h. A trans-BBB peptide was used as the positive control. Values are mean ± SEM of n = 3–6. *p < 0.05 vs positive control (one-way ANOVA followed by Dunnett post hoc tests). (d) BBB integrity to peptides 17 and 18. Measured as permeability of fluorescent FD4 dextran upon peptide exposure. Values are mean ± SEM of n = 3–6. No statistical significance difference was observed between samples (one-way ANOVA followed by Tukey’s multiple comparison test). (e) Confocal microscopy. Images showing peptide internalization into bEnd.3 cells.
