FIGURE 1.
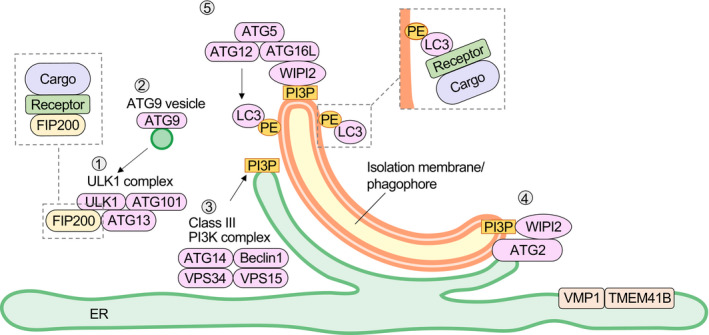
Autophagosome formation. When the ULK1 protein kinase complex1 is translocated to the ER subdomain, PI3K complex I (3) is recruited and the PI(3)P production increases. WIPI binds to PI(3)P and accumulates with its binding partner, ATG2 (4). ATG2 connects the ER to the isolation membrane/phagophore and transports lipids. ATG9, a membrane protein (2), transiently accumulates in the isolation membrane/phagophore and scrambles phospholipids transported by ATG2 from the ER to the cytoplasmic layer of the isolation membrane/phagophore. VMP1 and TMEM41B are ER membrane proteins that have been shown to be involved in regulating the cytoplasmic side of the ER. ATG12 and ATG5 are covalently bound to each other via a ubiquitin‐like conjugation reaction. The ATG12‐ATG5 conjugate forms a complex with ATG16L and localizes to the isolation membrane/phagophore (5), which determines the location of amide bond formation between ATG8 family proteins and PE. For selective autophagy, receptors that bind to both cargo and ATG proteins ensure selectivity. FIP200, a component of the ULK1 protein kinase complex and ATG9, interacts with these receptors to promote the formation of an isolation membrane/phagophore around the cargo. Interaction of these receptors with ATG8 family‐PE results in the elongation of the isolation membrane/phagophore along the substrate
