Fig. 4.
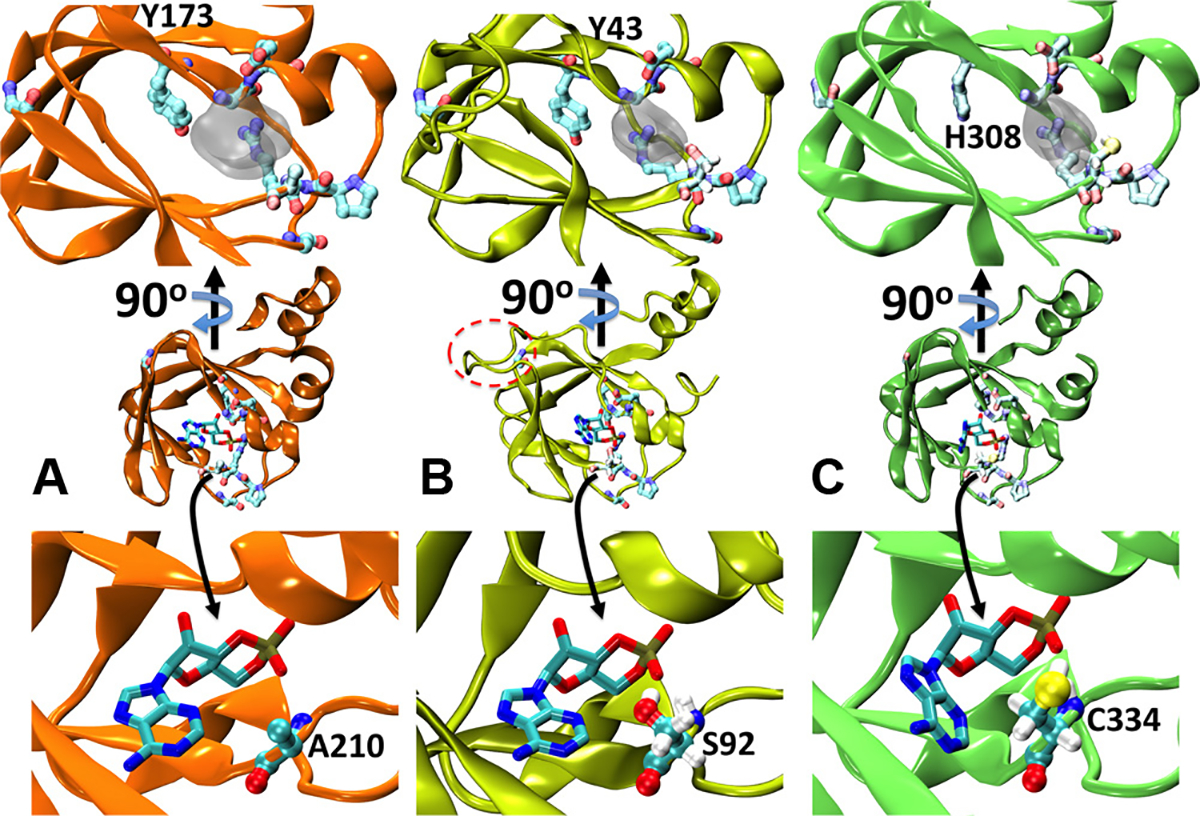
Structural modelling of T. cruzi’s cNMP binding proteins. (A) Cartoon representation of the X-ray structure of PKA-RIα (PDB id:3PNA). cAMP is shown as sticks, while the most conserved residues in Fig. 3 are presented as balls and sticks in pale colours. The inset on the top shows a different perspective on the binding site. For the sake of visual clarity, only the excluded volume of the ligand is indicated as semitransparent grey surface. (B) and (C) Homology models for TcCLB.508523.80 and TcCLB.510297.110, respectively. The label in the top insets indicates the position the tyrosine substituted by histidine in TcCLB.510297.110. The insets in the bottom depict the specific interaction between cAMP and alanine 210 in PKA-RIα (A), serine 92 in TcCLB.508523.80 (B) and cysteine 334 in TcCLB.510297.110 (C). In B, the extra loop between β strands 4 and 5 is highlighted with a dashed red oval. In B and C the hydrogen atoms of S92 and C334 are explicitly included to show the formation of a hydrogen bond with the phosphate moiety.
