FIGURE.
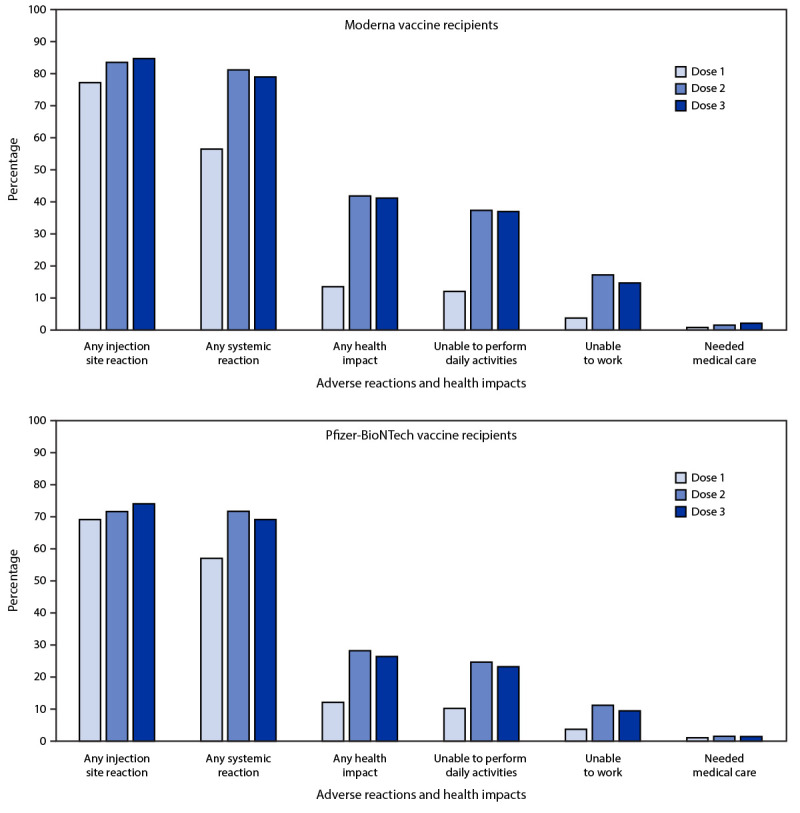
Adverse reactions and health impacts reported by persons who received 3 doses* of Moderna (N = 6,283) or Pfizer-BioNTech (N = 6,308) COVID-19 vaccine and completed at least one v-safe health check-in survey on days 0–7 after each dose, by dose number — United States, August 12–September 19, 2021
* The odds of reporting an event after dose 2 and 3 were compared using a multivariable generalized estimating equations model that accounted for the correlation between registrants and adjusted for demographic variables (receipt of care was not adjusted because of small numbers); p-values <0.05 were considered statistically significant. For Moderna recipients, all differences except any health impact and inability to perform daily activities were statistically significant. For Pfizer-BioNTech, all differences except the need for medical care were statistically significant.
