Abstract
COVID-19 pandemic raised a debate regarding the role of airborne transmission. Information regarding virus-laden aerosol concentrations is still scarce in community indoors and what are the risks for general public and the efficiency of restriction policies. This work investigates, for the first time in Italy, the presence of SARS-CoV-2 RNA in air samples collected in different community indoors (one train station, two food markets, one canteen, one shopping centre, one hair salon, and one pharmacy) in three Italian cities: metropolitan city of Venice (NE of Italy), Bologna (central Italy), and Lecce (SE of Italy). Air samples were collected during the maximum spread of the second wave of pandemic in Italy (November and December 2020). All collected samples tested negative for the presence of SARS-CoV-2, using both real-time RT-PCR and ddPCR, and no significant differences were observed comparing samples taken with and without customers. Modelling average concentrations, using influx of customers’ data and local epidemiological information, indicated low values (i.e. < 0.8 copies m−3 when cotton facemasks are used and even lower for surgical facemasks). The results, even if with some limitations, suggest that the restrictive policies enforced could effectively reduce the risk of airborne transmissions in the community indoor investigated, providing that physical distance is respected.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-021-16737-7.
Keywords: SARS-CoV-2, COVID-19, Airborne transmission, Coronavirus, Indoor, PCR
Introduction
The global pandemic due to COVID-19 disease, related to the coronavirus SARS-CoV-2, spread all around the world. By 31 December 2020, infected cases reached 82,401,782 individuals and 1,801,239 deaths worldwide (https://covid19.who.int/); 2,541,783 individuals were infected at the same date in Italy (about 4.3% of population) with 88,279 deaths. In most countries, a second wave of pandemic was observed in autumn 2020. In Italy, the second wave started roughly at the beginning of October 2020 and hit all regions contrarily to what happened during the first wave (winter 2020) in which northern Italian regions were severely hit compared to southern regions. This convinced the government to enforce mitigation strategies both at national and regional levels according to the estimated risks in the different regions.
Different mechanisms of SARS-CoV-2 transmission are widely accepted, but the role of airborne transmission in the spread of COVID-19 started a debate since the beginning of pandemic (Contini and Costabile 2020; Domingo et al. 2020; Klompas et al. 2020; Morawska and Cao 2020; Prather et al. 2020; Ram et al. 2021). Currently, even if a large number of studies are available, there are still several aspects not completely understood regarding risks of airborne transmission and the role of atmospheric conditions on this mechanism of transmission (Aleya et al. 2021; Borges et al. 2021; Escandón et al. 2021). Airborne transmission could be due to virus-laden particles emitted by infected individuals. Large droplets settle faster than they evaporate; in contrast, small droplets leave droplet nuclei (i.e. dry residuals after total or partial evaporation) (Asadi et al. 2020; Borouiba 2020). Droplet nuclei, containing virus aggregates, are aerosols that could stay suspended in air for long time, potentially contributing to airborne transmission (Allen and Marr 2020; Martano 2020; Morawska and Cao 2020; Tang et al. 2021).
One of the most important parameters to ascertain risks of airborne transmission is the concentration of virus-laden particles of different sizes in air (Chirizzi et al. 2021). However, there is a limited number of studies that investigate this aspect in real outdoor and indoor conditions, so that modelling is often applied (Buonanno et al. 2020; Feng et al. 2020; Lelieveld et al. 2020; Belosi et al. 2021). In residential outdoor conditions, with the exclusion of crowded areas or location near important sources like hospitals or quarantine areas, there is a number of evidences that air samples tested negative (i.e. concentrations under detection limits) or showed low concentrations of SARS-CoV-2 RNA in atmospheric particles of different sizes (Liu et al. 2020; Hu et al. 2020; Chirizzi et al. 2021; Dunker et al. 2021; Passos et al. 2021; Pivato et al. 2021). This suggests that the risk of airborne transmission of COVID-19 in outdoors is limited if physical distancing form relevant sources is maintained and crowds avoided. The same conclusion was obtained by the modelling results of outdoors concentration of virus-laden particles in Lombardy (northern Italy) during the first wave of pandemic (Belosi et al. 2021).
In indoor environments, most of the research efforts have been focused on measuring airborne viral particle concentrations in hospitals, intensive care units, and quarantine areas showing that concentrations could be higher compared to outdoor (Borges et al. 2021; Chia et al. 2020; Ge et al. 2020; Hu et al. 2020; Jin et al. 2020; Lednicky et al. 2020; Liu et al. 2020; Razzini et al. 2020; Santarpia et al. 2020; Passos et al. 2021; Vosoughi et al. 2021). However, other studies reported no detectable airborne viral RNA even in proximity of COVID-19 patients (Cheng et al. 2020; Faridi et al. 2020; Ong et al. 2020; Dumont-Leblond et al. 2021; Hemati et al. 2021). This underlines that there are difficulties in comparison of results obtained in different studies for several reasons: (i) the strength of the source (number of infected individuals); (ii) the absence of a standard measurement protocol (Borges et al. 2021; Pan et al. 2019; Robotto et al. 2021); (iii) the influence of specific mitigation strategies; (iv) the role of mechanical ventilation in the different indoors environments and the size of the rooms. For efficient reduction of the spread rate of COVID-19, it is important to assess risks of airborne transmission in community indoor environments, such as department stores, shopping centres, food supermarkets, public transport vehicles, restaurants, pharmacies, hair salons, schools and universities, and cinemas and theatres. In addition, it is relevant to understand if the COVID-19 contrasting measures applied in the different indoor environments (such as ventilation, filtration, use of facemasks, and limitations to the number of simultaneous customers) could effectively reduce these risks in different countries and different cities of the same country. These aspects are essential to plan future strategies or to correct ongoing strategies. Measurements of viral particle concentrations in real world conditions in these environments are very sparse so that, often, risks are evaluated with modelling approaches (Buonanno et al. 2020; Lelieveld et al. 2020; Xu et al. 2021). Recently, Xu et al. (2021) investigated the airborne infection risk of SARS-CoV-2 in 111,485 US public and private schools, evaluating the impacts of different mitigation strategies. Results indicated that, among the considered strategies, air filtration was the most effective, with average infection risk over 30% less than the risk levels estimated with the use of increased ventilation and hybrid learning strategies.
Some studies studied efficiency of airborne transmission of SARS-CoV-2 using detection and concentration measurements of virus traces in air samples. A recent study on a bus, during normal operations in Chieti (Central Italy), collected samples in the first wave of pandemic when restrictions policies were enforced and reported that all samples tested negative for the presence of SARS-CoV-2 (Di Carlo et al. 2020). The authors interpreted these negative results as a consequence of the restrictions on the maximum number of passengers, and the use of open windows during bus operation to increase ventilation that effectively reduced airborne transmission risks. In Moreno et al. (2021), six PM2.5 (i.e. particles with aerodynamic diameter < 2.5 μm) samples were collected in subway trains in June 2020. One sample was found positive for one of the three genetic targets of SARS-CoV-2 searched with RT-PCR and one sample for two genetic targets. Maximum estimated concentration was 23.4 copies m−3. In the same period (i.e. June 2020), also six air samples were collected in buses during normal operation, and one sample was found positive for one of the three genetic targets with a concentration of 1.4 copies m−3. This would suggest that airborne transmission in public transport could be an issue mainly in underground services. Hadei et al. (2021) investigated community indoor environments in Iran collecting 28 air samples, in different indoor sites that were found to be positive to the presence of SARS-CoV-2 in 64% of the cases, but no details on viral particle concentrations were reported. Correlation of positivity rates with the use of masks and the number of people were not statistically significant even if it was observed a slight increase with the number of people suggesting that limitations of customers allowed in indoors could reduce risk of airborne transmission.
This work tries to fill this gap in knowledge of concentrations of SARS-CoV-2 virus-laden particles in community indoors environments, for the first time in Italy, using measurements in different regions of Italy during the second wave of pandemic based on samples collected between 16 November and 21 December 2020. Measurements were compared with modelling results, done considering real conditions of the different environments, estimating the efficiency of the restrictive policies and of ventilation in reducing concentrations and, consequently, average risk of indoor airborne transmission.
Materials and methods
Sampling sites and measurement strategy
Aerosol samples were collected in different indoor community environments in three Italian regions: Veneto (North-Eastern Italy), Emilia-Romagna (central Italy), and Puglia (South-Eastern Italy) during the second wave of COVID-19 pandemic in the period between 16 November 2020 and 21 December 2020. These environments have been chosen because they were open also during lockdown or when restrictive measures applied (even if with some limitations) and were usually frequented by the public for everyday needs. A summary of the samples collected at the different sites, for the determination of the SARS-COV-2 genetic (RNA) material, is reported in Table 1. At all sites analysed some restrictions were enforced to limit COVID-19 spread. Specifically, it was compulsory to maintain the physical distancing, there were limits to the maximum number of people simultaneously present indoor, and access was allowed only with body temperature < 37.5 °C. It was compulsory to wear masks in all sites, with the exclusion of the canteen (site S4) in which masks were used to enter the premise and whenever a customer leaves the table, but masks were not used during lunches. Samples were collected using active sampling on quartz filters. This choice was done because it allowed using the methodology already developed for outdoor sites in a previous study (Chirizzi et al. 2021). In addition, sampling on filters is used in several studies that found both samples positive to SARS-CoV-2 (Kayalar et al. 2021; Setti et al. 2020) and samples negative (Chirizzi et al. 2021; Linillos-Pradillo et al. 2021; Passos et al. 2021; Pivato et al. 2021.
Table 1.
Summary of samples collected in the three regions with indication of the measurement periods, of the samples collected, and of the average sampling volume. Blank filters were used as control to check for unintentional contamination and for evaluation of recovery
| Area | Site | Period | Volume (m3) | Note | |
|---|---|---|---|---|---|
|
Venice-Mestre Veneto region |
S1 | Train station | 16–24 November | 29.2 | 14 PM10 samples (7 diurnal and 7 nocturnal) and 3 blank filters |
| S2 | Supermarket | 1–10 December | 29.7 | 6 PM10 diurnal samples and 3 blank filters | |
| S3 | Supermarket | 16–21 December | 29.6 | 9 PM10 samples (4 diurnal and 5 nocturnal) and 3 blank filters | |
|
Bologna Emilia-Romagna region |
S4 | Canteen | 19–26 November | 9.3 | 4 TSP samples, 2 background samples, and 2 blank filters |
|
Lecce Puglia region |
S5a | Shopping centre | 17–24 November | 22.9 | 14 PM10 filters (7 diurnal and 7 nocturnal) and 1 blank filter |
| S5b | Shopping centre | 14–21 December | 22.9 | 13 PM10 samples (6 diurnal and 7 nocturnal) and 2 blank filters | |
| S6 | Hair salon | 28 Nov.–04 Dec. | 6.2 | 4 TSP diurnal samples and 1 blank filter | |
| S7 | Pharmacy | 9–14 December | 6.5 | 5 TSP diurnal samples and 1 blank filter | |
In Veneto, PM10 samples were collected at three sites in the metropolitan city of Venice area using quartz fibre filters (Whatman, 47 mm in diameter), pre-fired at 400 °C in a muffle furnace, in an automatic sequential low-volume (38.3 L min−1, according to the EN 12341:2014 method) sampler (Skypost PM-TCR Tecora). The site S1 was the internal gallery of the Venice-Mestre train station (Fig. S1, supplementary material) in which samples were collected for about 12 h during the day (roughly between 7am and 7pm) and during the night (between 7pm and 7am) to compare periods with different numbers of travellers. During the pre-pandemic period, the train station was populated by about 80,000 people per day, and considering the decrease in the number of people (about 90%, data provided by Grandi Stazioni Rail), we can estimate a flow of 8000 people per day. The site S2 (Fig. S2, supplementary material) was the supermarket Interspar, in the city centre of Mestre-Venice, in which PM10 samples were collected during diurnal opening hours (roughly between 8am and 8pm). The volume of the supermarket was 18,861 m3 and the influx was about 2300 customers per day, with a total influx, during the sampling period, of 13,600 customers. The supermarket management estimated a decrease of the number of customers of about 25% compared to the pre-pandemic period, mainly due to lack of tourists. The site S3 was the supermarket Punta Gialla (A&O), located at Cavallino-Treporti, in the metropolitan city of Venice (Fig. S3), in which diurnal PM10 samples were taken during opening hours, roughly between 8am and 8pm. The influx was about 1500 of customers per day (total influx during the sampling period of about 6000 customers), and no significant reduction of customers was noticed by the supermarket management. The volume of the supermarket is 3520 m3 (1100 m2 of surface area for a height of 3.2 m), and it is equipped with a mechanical ventilation system.
In Emilia-Romagna, total suspended particle (TSP) samples were taken using quartz fibre filters (47 mm in diameter) with a low-volume (61.7 L min−1) sampler (Zambelli Explorer pump). Mass and particle number concentrations were obtained by means of an optical particle counter (Grimm, mod. 11-A). Measurements were taken in the canteen of the National Research Council (CNR), an environment (Fig. S4) of about 1700 m3 (500 m2 of surface area for a height of 3.4 m) equipped with a ventilation system with an airflow of 6250 m3/h able to perform about 3.7 air exchange per hour (AER). The average number of customers in the sampling period was 159 per day (range 144–172 per day). Access is allowed to a maximum of 28 people every 15 min. Each sample was taken for 2.5 h during distribution of lunches between 12am and 2:30pm, in addition, two background samples were taken between 9am and 12am (duration 2.5 h like the others) when the canteen was open, but before distribution of lunches.
In Puglia, samples were taken using quartz fibre filters (Whatman), pre-fired at 400 °C in a muffle furnace. The site S5a and S5b are the same indoor environment (commercial centre of Cavallino in Lecce) in which PM10 samples were collected with a low-volume (38.3 L min−1) sequential sampler (Zambelli Explorer Plus) in two different periods. The two measurement periods correspond to two different restrictive policies for containment of COVID-19 spread (see the “Epidemiological information and restriction policies during the measurements” section). In this site, samples were taken during diurnal opening hours (roughly between 9am and 9pm) and during nocturnal closure hours (between 9pm and 9am). The shopping centre includes 25 shops, 2 eating places, and 1 supermarket for a total volume of 14,448 m3. Mechanical ventilation insured a flow rate of 72,000 m3 h−1 with about 5 exchange of air per hour. The sampler was located in the gallery in front of the main entrance/exit of the supermarket (Fig. S5) where most of the customers are passing through. The shopping centre had a total influx of about 30,000 customers during the sampling period S5a that raised to about 80,000 in the sampling period S5b. The maximum number of people simultaneously inside was of about 1400 customers. The site S6 (Fig. S6) was a hair salon (Lounge Parrucchieri Philosophy) in Lecce with a volume of 280 m3 relying on natural ventilation (also maintaining the doors open during pandemic) and with a maximum number of three customers simultaneously allowed inside. During the measurement period, it had an average of 4 customers per day with an estimated reduction of 30% compared to the pre-pandemic period. In this site, samples were collected with an in-house built manual TSP sampler operating at low-volume (13.4 L min−1) during opening hours roughly between 9:30am and 5pm. The site S7 was a pharmacy (Fig. S7) in Lecce (Moschettini) with a volume of 410 m3 and a maximum number of six customers simultaneously allowed inside. Mechanical ventilation insured 920 m3 h−1 for about 2.2 air exchanges per hour. In site S7, samples were taken during opening hours, roughly between 9:30am and 5:30pm. The average number of daily customers during the measurements was 280 (range 252–305) with no significant changes compared to the same period of the year 2019.
In some previous studies, indoor sampling was done for different size fractions. Liu et al. (2020) collected air samples in indoor environments in hospitals in China and detected SARS-CoV-2 RNA in the size range 0.25–1 μm and in the range >2.5 μm. The indoor measurements in Singapore hospitals (Chia et al. 2020) showed that the smallest aerodynamic size fraction that contained detectable levels of SARS-CoV-2 was 1–4 μm but the virus was detectable also in particles of sizes >4 μm. Stern et al. (2021) collected samples in a Boston (Massachusetts, USA) hospital in three size ranges (> 10 μm, 10–2.5 μm, and <2.5 μm) finding that SARS-CoV-2 genetic material was present in all size fractions. Therefore, the choice to sample PM10 and TSP, that includes all size ranges mentioned, appears to be adequate to detect eventual presence of SARS-CoV-2 in air.
The sampling volume is different from site to site depending on both, the flow-rate of the sampler used and the opening hours of the site studied. The average sampling volumes used at the different sites (Table 1) are comparable, or even larger, with the values used in several other studies focused on determination of airborne SARS-CoV-2 in indoor environments. For example, Stern et al. (2021) sampled different size fractions at 5 L min−1 for 14.4 m3 of total volume; Moreno et al. (2021) sampled PM2.5 at 10 L min−1 for volume ranging between 5.2 m3 and 6.2 m3; Di Carlo et al. (2020) used 24 L min−1 to sample 18.7 m3 of TSP; Razzini et al. (2020) sampled TSP at 50 L min−1 for total volumes of 2 m3; Hadei et al. (2021) used two samplers at different flow rate for TSP sampling between 0.2 and 3.5 m3; Passos et al. (2021) used low-volume and high-volume samplers in different sites with sampled volumes ranging from 0.12 up to 120 m3.
After sampling, all samples were frozen, at −25 °C for the site in Emilia-Romagna region and at −80 °C for the sites in Veneto and Puglia regions, immediately after sampling for conservation until the successive analysis.
Analytical method for RNA detection
The approach and methodology used is essentially the same used for a previous study on outdoor samples (Chirizzi et al. 2021). RNA extraction was done using Total RNA Purification Kit (Norgen Biotek Corp.) with a modified protocol to increase yield. Each filter was cut and placed inside a 2-mL centrifuge tube containing 1-mL of phosphate buffer solution (PBS, pH 7.4). The tube was sealed and treated in a sonicator bath for 30 min. Particle deposit was separated by centrifugation using a mini syringe placed in a collection tube. The pellet obtained was resuspended in 350 μL of supernatant, according to the manufacture’s protocol. The final eluted solutions (between 60 and 70 μL in total) were frozen at −80 ± 2 °C and stored until analysis.
Molecular analysis for the detection of SARS-CoV-2 was carried out using real-time RT-PCR and droplet digital PCR (ddPCR) technologies. According to World Health Organization (WHO), real-time RT-PCR represents the gold standard for the diagnosis of SARS-CoV-2; however, ddPCR has demonstrated the best performance to detect SARS-CoV-2, because it reduces the false negatives with a high sensitivity and accuracy of 95.5% (Suo et al. 2020; Dong et al. 2021). In the present study, real-time RT-PCR for SARS-CoV-2 was carried out on a CFX96TM Real-Time system (Bio-Rad, Italy) using GeneFinderTM COVID-19 Plus RealAmp Kit (OSANG Healthcare, Korea). The GeneFinderTM COVID-19 Plus RealAmp Kit is a One-Step real-time reverse transcription (RT-PCR) multiplex assay based on fluorescent-labelled probe used to confirm the presence of SARS-CoV-2 RNA by amplification of RdRp, N, and E genes. The kit provides all the reagents required for the analysis, PCR positive and PCR negative controls included. Five microliters of extracted RNA was added to 15 μL of master mix for each sample and analysed according to kit instructions. Undiluted and 1:10 diluted samples were tested. In each run, two negative controls (molecular grade water) and a positive control were added. The interpretation of the sample results was done according to kit instructions. In detail, a sample was considered positive for the presence of SARS-CoV-2 when the Ct for at least one or both of the RdRp or N genes was ≤ 40. The limit of detection (LOD) of the GeneFinderTM COVID-19 Plus RealAmp Kit was declared by the manufacturer in 10 copies/reaction, equal to 2 copies/μl for all genes.
The ddPCR assay was performed according to the protocol described by Alteri et al. (2020) on QX200TM Droplet DigitalTM PCR system (Bio-Rad, Italy). The assay is a home-made reverse transcription (RT) droplet digital PCR (ddPCR) protocol designed for the quantitative detection of SARS-CoV-2 RNA with the One-Step RT-ddPCR Advanced Kit for Probes (Bio-Rad, Italy). The method consists in a duplex targeting the RNA dependent RNA polymerase (RdRp) of SARS-CoV-2 and the housekeeping gene RNAse P, as reported by CDC, into a single assay multiplex to enable a one-well reaction. The reaction mixtures were separated into approximately 20,000 droplets using a QX200 Droplet GeneratorTM (Bio-Rad, Italy) with random dispersal of target nucleic acids into the droplets. The PCR assays were performed in a C1000 TouchTM Thermal Cycler (Bio-Rad, Italy). After amplification, the droplets were individually assayed using the QX200TM Droplet ReaderTM. The fluorescence data were then analysed by the QuantaSoft v1.7 Software (Bio-Rad, Italy) to determine the presence of SARS-CoV-2 in the specimen. The LOD of the ddPCR assay was previously estimated by Alteri et al. (2020) in 2.9 copies/reaction, equal to 0.58 copies/μL. For comparison, the methodology used by Liu et al. (2020) had a LOD of 2.18 copies/reaction and that used by Pivato et al. (2021) had a LOD of 2.5 copies/μL.
Analysis of RNA recovery
The efficiency of the extraction and sample conservation procedures was estimated evaluating the recovery of a process control; a virus appositely added on filters prior to acid nucleic extraction. Mengovirus strain MC0, supplied by Istituto Superiore di Sanità (ISS, Rome, Italy), was used as process control. The efficiency of the extraction method was evaluated comparing the Ct values obtained for Mengovirus on samples extracts with a reference. In detail, 10 μL of Mengovirus was added to (i) 1 mL of PBS (reference sample); (ii) 1 mL of PBS with a blank filter; and (iii) 1 mL of PBS in an environmental filter. The detection of Mengovirus was done using a CFX96TM Real-Time system (Bio-Rad, Italy) using amplification conditions, primers, and probe and reagents RNA UltraSenseTM One-Step Quantitative RT-PCR System (Life Technologies, Carlsbad, CA, USA) (Pintó et al. 2009).
Different cases were analysed for recovery analysis: one set of filters were spiked with Mengovirus in laboratory and directly extracted; a second set of filters were spiked and frozen at −25 °C and successively extracted with the other samples; a third set of filters were spiked and mounted in one of the samplers (the PM10 sampler in Lecce without effective sampling on them). Results indicate a recovery of 69% (± 8% standard error) for the first set of filters immediately extracted after spiking (with no effects of sample conservation). The recovery of the second set of filters, those conserved at −25 °C, gave a recovery of 62% (± 14% standard error), thereby comparable with the first set. This means that conservation at −25 °C did not significantly change the recovery. The third set gave a slightly lower recovery, equal to 54% (± 13% standard error), suggesting that the use of filters inside the sampler reduces the recovery. This value is in agreement with that observed in a previous study (Chirizzi et al. 2021). Our recovery is lower than the recovery (almost 100%) claimed in Passos et al. (2021) after a test on different sampling substrates that behaved similarly (cellulose nitrate filters, PTFE filters, and quartz filters). On the other hand, it is larger than the recovery (about 20%) observed in Hadei et al. (2021) with sampling on PTFE filters. It must be said that the relatively large standard errors in our data indicate a variability likely due to a matrix effect, for example residual fibres of the filters extracted during the procedure. Another aspect is that conservation at room temperature seems to significantly decrease the recovery, even if systematic tests are not available. Therefore, in order to improve detection of SARS-CoV-2 genetic material and to make more comparable results obtained in different studies, it would be necessary to test recovery in different conditions and over different substrates developing an opportune protocol for sampling and conservation of samples. The necessity of a standardised protocol for sampling, conservation of samples, and analysis, is also mentioned by Yun et al. (2020), Borges et al. (2021), and by Robotto et al. (2021).
Epidemiological information and restriction policies during the measurements
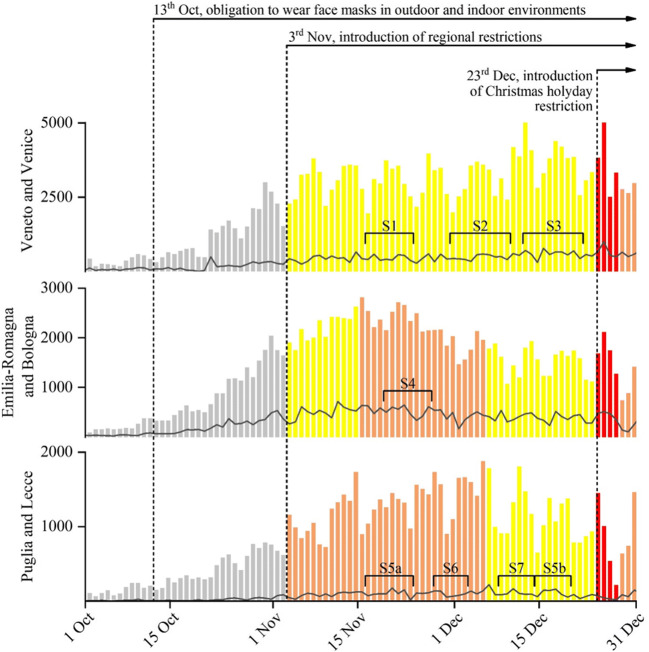
During autumn 2020, starting roughly at the beginning of October, a second wave of pandemic hit Italy. It spread all over Italy even if with different intensity in the different regions. This leads to the introduction of restrictive policies at national and regional levels in different periods (Fig. 1) in addition to the social distancing and no crowd measures already enforced since winter 2020. Specifically, on 13 October, at national level, the use of facemasks in outdoor and indoor environments, the closure of gyms and pools, the closure of congresses and limitations for parties and religious ceremonies, and the closure of discos were enforced. Limitation for restaurants and pub were enforced favouring delivery and take away. In addition, limitation to schools and university activities with use of distance learning was enforced. On 3rd November, regional restrictions were enforced on the basis of three “colours” (i.e. yellow, orange, and red) that correspond to different risk of the spread of contagion. In red regions (i.e. maximum risk), movements of people were not allowed (with exception for work, health, and emergency reasons); shops were closed with the exclusion of necessary shops (such as food markets); and restaurants were closed being allowed only delivery of take away until 10pm. In orange regions (i.e. intermediate risk), movements of people were allowed in the same municipality between 5am and 10pm, and shops were opened with the exclusion of restaurants (could operate only with delivery or take away) and commercial centres that are closed during weekends (and holydays). In yellow regions (i.e. low risk), the movements of peoples were allowed between 5am and 10pm, shops were opened including restaurants and bars (but limited to 6pm, afterwards only delivery or take away), and commercial centres closed during weekends and holydays with the exclusion of food markets.
Fig. 1.
Daily new coronavirus cases in Veneto, Emilia-Romagna, and Puglia regions (coloured bars) and in Venice, Bologna, and Lecce metropolitan areas (grey lines). Colours of the bar indicate different regional restrictive containment measures enforced. The different sampling periods are also reported with square brackets
Figure 1 reports the daily number of new contagions (source, Italian Ministry of Health) in the three regions (bars) and in the three metropolitan areas (lines) in which samples were taken. The colour of the bars indicates the restrictions that were active during the different periods. Measurements in Venice (for sites S1, S2, and S3) were done in yellow periods; the site S4 in Bologna was studied in orange period; samples in site S5 in Lecce were taken in both orange period (S5a) and yellow period (S5b) for comparison. Site S6 was studied in orange period and site S7 in yellow period.
Discussion of results
All collected samples tested negative for the presence of SARS-CoV-2 genetic material, with both analysis real-time RT-PCR and ddPCR, either for samples collected during opening hours and for samples collected during closure hours (i.e. absence of customers). This means either that the SARS-CoV-2 genetic material is absent in the samples taken or that the concentrations are lower than the detection threshold of the methodology used. The LOD (genome copies μL−1) was transformed in detection thresholds for atmospheric concentrations of viral particles (expressed in copies m−3) estimating the threshold of viral particles contained in a single filter (i.e. RNA copies per filter). This was done considering the total volume of extraction solutions. Results were successively normalised by the average sampled volume in each condition to obtain the thresholds. In sites S1, S2, and S3, located in Venice area, the estimated concentrations of virus-laden particles in PM10 were < 1.3 copies m−3. In site S4 in Bologna area, the estimated concentrations of virus-laden particles in TSP were < 4 copies m−3. In site S5a and S5b in Lecce area the estimated concentrations of virus-laden particles were < 1.5 copies m−3 and < 5.5 copies m−3 for sites S6 and S7. The differences in the thresholds of the various sites (summarised in Table 2) are due to the different sampling times and flow rates being the LOD constant. Thresholds are not often reported in previous studies; however, these values are comparable, for example, with the detection limit of 1.2 copies m−3 reported by Pivato et al. (2021).
Table 2.
Summary of results obtained at the different sites, including main characteristics of the sites: indoors volumes, air exchange rates due to mechanical ventilation, and average number of customers per day. The number of active cases (i.e. persons currently infected) per thousand of inhabitants in the different areas and the expected number of customers infected per day are also reported. Finally, the LOD of measurements and the results of simulations are reported
| Venice | Bologna | Lecce | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | S1 | S2 | S3 | S4 | S5 | S6 | S7 | ||
| Volume (m3) | - | 18,861 | 3520 | 1700 | 14,448 | 280 | 410 | ||
| AER (h−1) | - | 1.6 | 1.6 | 3.7 | 5 | Natural | 2.2 | ||
| N. customers (day−1) | 8000 | 2300 | 1500 | 159 | 5a | 30,000 | 4 | 280 | |
| 5b | 80,000 | ||||||||
| Active cases/1000 inhabitants | 12 | 12 | 12 | 16 | 5a | 2.9 | 2.9 | 2.9 | |
| 5b | 2.2 | ||||||||
| N. infected customers (day−1) | 96 | 28 | 18 | 2 | 5a | 87 | ~ 0 | 1 | |
| 5b | 176 | ||||||||
| RT-PCR and ddPCR results | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | ||
| LOD (copies m−3) | <1.3 | <1.3 | <1.3 | <4 | <1.5 | <5.5 | <5.5 | ||
| Simulations (copies m−3) | No mask | - | 0.5 | 1.8 | 0.6 | 0.4 | - | 0.8 | |
| Surgical mask | - | 0.01 | 0.04 | - | 0.01 | - | 0.02 | ||
| Cotton mask | - | 0.2 | 0.8 | - | 0.16 | - | 0.3 | ||
To better understand if the conditions at the different sites, including the epidemiological situations of the different areas, are compatible with negative results, the average expected concentrations in the different indoor environments were investigated making some assumptions on emission rate (E) of virus-laden aerosol from infected individuals, on the number of expected infected customers (n), and on the distribution of particles in the different environments considering their volumes and their ventilation characteristics. When a continuous source of particles is activated at time t=0, in an otherwise clean room of volume V, having a ventilation airflow equal to Q, the concentration C(t) could be expressed, neglecting dry deposition, by Bhagat et al. (2020):
| 1 |
where is the time constant of the structure (equal to the inverse of the air exchange rate defined in the “Sampling sites and measurement strategy” section). For t ≫ τ, a steady concentration is reached (a period of 3τ is sufficient to reach 95% of C0). When the source is turned off, for example the infected individuals leave the room, the concentration will decrease from the initial value Ci that has been reached before the cutoff of the source:
| 2 |
Previous equations are based on the assumption that the air is well-mixed within the modelled space and particles are instantaneously and evenly distributed in the room (Gammaitoni and Nucci 1997). This approach is reasonable when details of geometries and airflows in the different microenvironments are not known, and it has been previously applied to study indoor risk of airborne SARS-CoV-2 (Buonanno et al. 2020). There could be some uncertainties associated with the assumption that the air is well-mixed and particles are instantaneously and evenly distributed in the room, because, for example, concentrations might be higher in poorly ventilated parts of the room.
The emission E of viral RNA copies, without use of masks, can be estimated using a mass balance approach between the viral load in mouth (CV) and the volume of particles emitted during respiration and speaking, as done in previous studies (Buonanno et al. 2020; Belosi et al. 2021). An average E of 7000 copies h−1 is evaluated assuming Cv=109 copies mL−1 (Buonanno et al. 2020); and assuming the size distribution of respiration droplets, divided in four size ranges (Morawska et al. 2009) and averaged between unmodulated vocalization and voiced counting; the inhalation rate for light exercise, averaged between male and female, equal to 1.38 m3 h−1 (Adams 1993). The emission rate used in these simulations is an average value already used in several other studies; there could be a high variability of the viral load and a strong dependency on the day of detection compared to the onset of COVID-19. In addition, there could be super-spreaders which have much higher emission rate that can create more critical situation sin indoor environments. The results of Eqs. (1) and (2) are linearly dependent on the emission rates so that the calculated concentrations will linearly increase with average emission rate. This emission rate is reduced by the use of facemasks. The filtration efficiency, for removal of particles, of every-day masks depends on eventual leakages, on the type of mask, and on the size of particles. We consider two types of masks, widely used by the public in everyday life: surgical masks and cotton masks. For surgical masks, the efficiency (Balachandar et al. 2020) for the four size ranges of respiratory particles considered here (i.e. the same range from 0.9 (at diameter of 0.8 μm) to 0.99 (at diameter of 5.5 μm); for cotton masks the efficiency ranges from 0.1 to 0.7 in the same size ranges (Balachandar et al. 2020). The reduction on emitted viral load depends also on content of virus-laden aerosol in each one of the four size range that could be evaluated with the mentioned mass balance approach (Buonanno et al. 2020). Considering the viral load in each of the four size ranges, the cumulative efficiency in reduction of E would be 98% for surgical masks and 58% for the cotton masks. Equations (1) and (2) could be used for estimating expected concentrations in the sites studied, taking into account the epidemiological conditions of the three cities at the time of sampling, in order to compare with the results obtained in collected air samples.
The epidemiological analysis of the Venice area indicates an average number of currently infected people per thousand of inhabitants roughly equal to 12 during the sampling period (source, Regional Health Agency of Veneto). Considering the average daily number of customers at site S2, about 2300 distributed in the opening hours, it is reasonable to assume an average of about 2.3 infected individuals per hour inside. Details on ventilation are not known for this site; however, it could be estimated a reasonable airflow considering the guideline UNI10339 of 2014 in which an airflow for medium ventilation of 7 L min−1 per person is suggested for these environments calculated using an occupancy of 0.2 person m−2. For the case of sites S2 and S3, an air exchange rate of 1.6 per hour is obtained. The expected steady-state average concentrations for site S2 will be 0.01copies m−3 using surgical masks and 0.2 copies m−3 using cotton masks. The expected average concentration will rise to 0.5 copies m−3 if use of facemasks would not be enforced. For site S3, using the same value of the air exchange rate of site S2 and the average expected number of 1.5 infected individuals per hour inside, a steady state average concentration of 0.04 copies m−3 is obtained considering surgical masks and of 0.8 copies m−3 considering cotton masks. The expected average concentration will rise to 1.8 copies m−3 if use of facemasks would not be enforced. These are low concentrations, especially considering that surgical masks are the most diffused in Italy, compatible with the negative results found on collected samples.
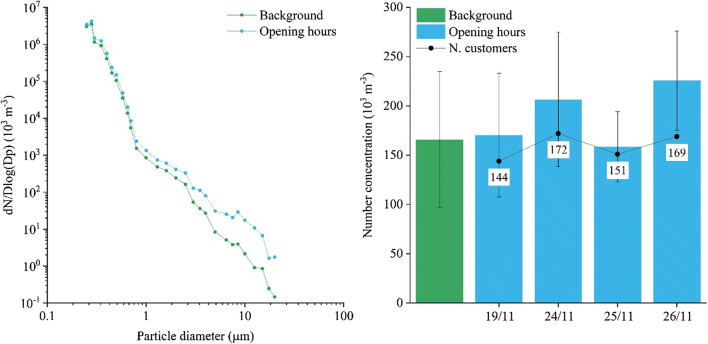
The epidemiological data of Emilia-Romagna region indicated an average number of currently infected people per thousand of inhabitant equal to 16 during the sampling period at site S4 (source, Regional Health Agency of Emilia-Romagna). This regional data has been preferred in comparison to metropolitan value, for this site, because the canteen is used by CNR employees and a relevant fraction of them live in different towns and provinces of Emilia-Romagna region. Considering the average number of customers (159 per day), it is reasonable to expect, on average, 2 infected customers per day. Taking into account an average permanence time of 30 min, it is possible to think at two scenarios: the first is with two infected customers present in the same shift; the second is with one infected customer in two successive shifts. Using previous equations in the first scenario, the average concentrations in the sampling period will be 0.6 copies m−3 (the maximum will be 1.9 copies m−3); in this case, concentrations are obtained without use of facemasks. The maximum concentration is evaluated at the end of the concentration growing period (Eq. 1) equal to 30 min (i.e. the permanence time in scenario 1). The second scenario has a similar average but a lower maximum (1.1 copies m−3 after 60 min). In both cases, the average concentrations are quite low and compatible with the negative results obtained in collected samples. Size distributions at site S4 during opening hours and background periods are compared in Fig. 2 that shows an increase of particles concentration mainly for particles with D > 1 μm. By comparison, the average concentration of these large particles in the different sampling periods ranges between 158,000 m−3 and 225,000 m−3 (average 190,000 m−3), as shown in Fig. 2.
Fig. 2.
Size distributions of particles in site S4 during opening hours and during background conditions (absence of customers). Number concentrations of particles with D>1 μm during the four sampling periods at site S4 and the average background value. The number of daily customers is included
The epidemiological data of Lecce municipality indicated an average number of currently infected people per thousand of inhabitants equal to 2.9 during the sampling period S5a and equal to 2.2 during the sampling period S5b (source, Regional Health Agency of Lecce). These numbers are essentially the same at province level. In the worst conditions at the commercial centre studied in Lecce (S5a and S5b), 1400 customers were counted simultaneously present inside with an average permanence time variable between 30 and 60 min. This time is significantly larger than the time constant of the structure (about 12 min) so that it is reasonable to assume that an equilibrium concentration will be reached in these conditions. This will be 0.01 copies m−3 when surgical masks are considered, about 0.16 copies m−3 when cotton masks are considered, and 0.4 copies m−3 when facemasks are not used. These are very low concentrations, even if evaluated in the worst conditions, compatible with the negative tests found on PM10 collected samples. The number of daily customers for the hair salon (site S6) is small; the probability to have an infected person during the sampling period is about 4%; thereby, it is reasonable to think that the negative results could also be due to the absence of infected people inside during the measurement period. It has also to be mentioned that customers are usually received with an agreed scheduling so that the probability to have two people inside simultaneously is essentially negligible. In site S7, considering the average number of customers per day, there is a probability of 70% to have one infected customer in each day of measurements.
Assuming an average permanence time of 10 min, this will give an estimated average concentration, in the period of 90 min necessary to reach maximum concentration (10 min) and decreasing back to 5% of the maximum (80 min) of 0.02 copies m−3 when surgical masks are considered, 0.3 copies m−3 for cotton masks, and 0.8 copies m−3 if facemasks are not used. These concentrations are effectively low and compatible with the negative results found on collected samples.
Results of measurements and simulations for the different sites are summarised in Table 2 to allow a better comparison.
Limitations and uncertainties of this study
Currently, there is not a standard protocol for the study of airborne SARS-CoV-2, and this leads to limited ability to compare results from different studies. The protocol used in this work was developed based on previous experience on outdoor detection of SARS-CoV-2 (Chirizzi et al. 2021) and on the expertise of available studies published since the beginning of pandemic.
The negative results found on collected samples are not uncommon also in other published indoor studies; the recent review by Borges et al. (2021), on detection of SARS-CoV-2 in indoor environments, shows that negative results were found in 10 studies (out of 25), while 15 of the studies showed positive results in at least one sample. Our results mean that concentrations of SARS-CoV-2 RNA in the studied sites and in the specific sampling periods are lower that the detection limits (LODs) reported in Table 2. It is possible that, lowering the detection limits, for example with larger sampling volumes, the virus could have been detected; however, concentrations would have been lower that the indicated LODs.
We do not know exactly how many infected customers were present at the different sites during sampling, and it is also possible that no infected people were present. However, the epidemiological situations of the different studied area, together with the detected number of customers, would suggest that the possibility to have some infected customers is not negligible (Table 2). In addition, it should be mentioned that samples were collected in a limited number of indoor environments and in specific periods so that they do not exclude that there could be other indoors, for example small and poorly ventilated, or other periods more critical regarding airborne concentrations of SARS-CoV-2.
Conclusions
The presence of airborne SARS-CoV-2 RNA was investigated in different community indoor environments (one train station, one commercial centre, two supermarkets, one canteen, one hair salon, and one pharmacy) in three Italian metropolitan areas: Venice (Veneto Region, NE of Italy), Bologna (Emilia-Romagna region, central Italy), and Lecce (Puglia region, SE of Italy). During the sampling periods at the different sites, national and regional measures to contrast COVID-19 spreads were enforced. In the different sites, 59 air samples were collected during diurnal hours when customers were present in the different indoor environments, and 23 background samples were taken in absence of customers. All collected air samples tested negative for the presence of SARS-CoV-2, and no significant differences were observed comparing samples taken with and without customers in the different indoor environments studied, meaning that SARS-CoV-2 RNA concentrations were lower than the detection limits of the protocols used (Table 2). Modelling of expected average concentrations in the different environments, done using influx of customers data and local epidemiological information, indicated low values (i.e. < 0.04 copies m−3 when surgical masks are used and < 0.8 copies m−3 when cotton masks are used) for the different sites with the exclusion of the canteen in which no facemasks are used during the lunch and average concentration of 0.6 copies m−3 could be estimated. These values are compatible with the negative results obtained in collected samples.
The results, even if with the limitation stated in the previous section, suggest that mechanical ventilations and limitation to the number of customers are very important aspects for reducing airborne concentrations of SARS-CoV-2 and the same apply for the use of facemasks. These are aspects targeted by restrictive measures adopted at both national and regional levels, and results suggest that they are important for reducing virus-laden particle concentrations in the studied community indoor environments, reducing, consequently, the risks for airborne transmission, providing that physical distance is maintained.
However, further studies are needed to improve air sampling technologies that can detect the presence of aerosolized viruses, effectively collect them and maintain their viability, and determine their distribution in aerosol particles.
Supplementary Information
(DOCX 4644 kb)
Acknowledgements
This study was performed within the cooperation project AIR-CoV (evaluation of the concentration and size distribution of SARS-CoV-2 in air in outdoor and indoor environments). Authors wish to thank all the structures that hosted our sampling instruments and, specifically, Sig. Francesco Vanin (Punta Gialla Srl); Sig. Filippo Boggian (Aspiag Service); Sig. Cristian Bottazzo (Lounge Parrucchieri Philosophy); Dr. Maria Rosaria Frigino and Dr. Massimo Natali (Farmacia Moschettini); Dr. Antonio Giannone (Klepierre Management Italia S.r.l) manager of the commercial centre of Cavallino in Lecce; Grandi Stazioni Rail; and Punta Gialla supermarket (A&O); the Presidency and the Technical Direction of the Research Area of the CNR of Bologna.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contribution
D. Contini, A. Gambaro, F. Belosi, and G. La Salandra conceptualized the study design; E. Barbaro, E. Gregoris, and M. Feltracco collected samples in Veneto and contributed to data post-processing; M. Conte and A. Dinoi collected samples in Puglia and contributed to data post-processing; S. Trabucco collected samples in Emilia-Romagna and contributed to data post-processing; D. Chirizzi, G. Ciccarese, G. La Salandra, and G. La Bella carried out the laboratory tests. All authors collaborated to interpretation of results, wrote, read, commented, and approved the final manuscript.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams WC (1993) Measurement of breathing rate and volume in routinely performed daily activities. Final report. Human Performance Laboratory, Physical Education Department, University of California, Davis. Human Performance Laboratory, Physical Education Department, University of California, Davis. Prepared for the California Air Resources Board, Contract No. A033-205, April 1993.
- Aleya L, Gu W, Howard S (2021) Environmental factors and the epidemics of COVID-19. Environmental Science and Pollution Research. 10.1007/s11356-021-14721-9 [DOI] [PMC free article] [PubMed]
- Alteri C, Cento V, Antonello M, Colagrossi L, Merli M, Ughi N, Renica S, Matarazzo E, Di Ruscio F, Tartaglione L, Colombo J, Grimaldi C, Carta S, Nava A, Costabile V, Baiguera C, Campisi D, Fanti D, Vismara C et al (2020) Detection and quantification of SARS-CoV-2 by droplet digital PCR in real-time PCR negative nasopharyngeal swabs from suspected COVID-19 patients. PLoS ONE 15(9):e0236311. 10.1371/journal.pone.0236311 [DOI] [PMC free article] [PubMed]
- Asadi S, Bouvier N, Wexler AS, Ristenpart WD. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Science and Technology. 2020;54(6):635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar S, Zaleski S, Soldati A, Ahmadi G, Borouiba L. Host-to-host airborne transmission as a multiphase problem for science-based social distance guidelines. International Journal of Multiphase Flow. 2020;132:103439. doi: 10.1016/j.ijmultiphaseflow.2020.103439. [DOI] [Google Scholar]
- Bhagat RK, Wykes MSD, Dalziel SB, Linden PF (2020) Effects of ventilation on the indoor spread of COVID-19. Journal of Fluid Mechanics 903(F1) [DOI] [PMC free article] [PubMed]
- Allen JG, Marr LC. Recognizing and controlling airborne transmission of SARSCoV-2 in indoor environments. Indoor Air. 2020;30:557–558. doi: 10.1111/ina.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosi F, Conte M, Gianelle V, Santachiara G, Contini D. On the concentration of SARS-CoV-2 in outdoor air and the interaction with pre-existing atmospheric particles. Environmental Research. 2021;193:110603. doi: 10.1016/j.envres.2020.110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges JT, Nakada LY, Maniero MG, Guimarães JR (2021) SARS-CoV-2: a systematic review of indoor air sampling for virus detection. Environmental Science and Pollution Research. 10.1007/s11356-021-13001-w [DOI] [PMC free article] [PubMed]
- Borouiba L. Turbulent gas clouds and respiratory pathogen emissions, potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Buonanno G, Stabile L, Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environment International. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VCC, Wong SC, Chan VWM, So SYC, Chen JHK, Yip CCY, Chan KH, Chu H, Chung TWH, Sridhar S, To, K.K.W. Chan JFW, Hung IFN, Ho PL, Yuen KY. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19) Infection Control & Hospital Epidemiology. 2020;41(11):1258–1265. doi: 10.1017/ice.2020.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Lim XF, Lim AS, Sutjipto S, Lee PH, Son TT, Young BE, Milton DK, Gray GC, Schuster S, Barkham T, De PP, Vasoo S, Chan M et al (2020) Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications 11:2800 [DOI] [PMC free article] [PubMed]
- Chirizzi D, Conte M, Feltracco M, Dinoi A, Gregoris E, Barbaro E, La Bella G, Ciccarese G, La Salandra G, Gambaro A, Contini D. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environment International. 2021;146:106255. doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D, Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020;11:377. doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- Di Carlo P, Chiacchiaretta P, Sinjari B, Aruffo E, Stuppia L, De Laurenzi V, Di Tomo P, Pelusi L, Potenza F, Veronese A, Vecchiet J, Falasca K, Ucciferri C. Air and surface measurements of SARS-CoV-2 inside a bus during normal operation. PLoS ONE. 2020;15(11):e0235943. doi: 10.1371/journal.pone.0235943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Marquès M, Rovira J. Influence of airborne transmission of SARS-CoV-2 in COVID-19 pandemic. A review. Environmental Research. 2020;188:109861. doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2. Theoretical considerations and available evidence. JAMA. 2020;324(5):441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- Dong L, Zhou J, Niu C, Wang Q, Pan Y, Sheng S, Wang X, Zhang Y, Yang J, Liu M, Zhao Y, Zhang X, Zhu T, Peng T, Xie J, Gao Y, Wang D, Dai X, Fang X. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224:121726. doi: 10.1016/j.talanta.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont-Leblond N, Veillette M, Bhérer L, Boissoneault K, Mubareka S, Yip L, Dubuis ME, Longtin Y, Jouvet P, McGeer A, Duchaine C (2021) Positive no-touch surfaces and undetectable SARS-CoV-2 aerosols in long-term care facilities: an attempt to understand the contributing factors and the importance of timing in air sampling campaigns. American Journal of Infection Control. 10.1016/j.ajic.2021.02.004 [DOI] [PMC free article] [PubMed]
- Dunker S, Hornick T, Szczepankiewicz G, Maier M, Bastl M, Bumberger J, Treudler R, Liebert UG, Simon JC. No SARS-CoV-2 detected in air samples (pollen and particulate matter) in Leipzig during the first spread. Science of the Total Environment. 2021;755:142881. doi: 10.1016/j.scitotenv.2020.142881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escandón K, Rasmussen AL, Bogoch II, Murray EJ, Escandón K, Popescu SV, Kindrachuk J (2021) COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection.BMC Infectious Diseases 21, 710. [DOI] [PMC free article] [PubMed]
- Faridi S, Niazi S, Sadeghi K, Naddafi K, Yavarian J, Shamsipour M, Jandaghi NZS, Sadeghniiat K, Nabizadeh R, Yunesian M, Momeniha F, Mokamel A, Hassanvand MS, Azad TM. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Science of the Total Environment. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Thierry M, Sperry T, Yi H. Influence of wind and relative humidity on the social distancing effectiveness to prevent COVID-19 airborne transmission: a numerical study. Journal of Aerosol Science. 2020;147:105585. doi: 10.1016/j.jaerosci.2020.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammaitoni L, Nucci MC. Using a mathematical model to evaluate the efficacy of TB ontrol measures. Emerging Infectious Diseases. 1997;3:335–342. doi: 10.3201/eid0303.970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X-Y, Pu Y, Liao C-H, Huang W-F, Zeng Q, Zhou H, Yi B, Wang A-I, Dou Q-Y, Zhou P-C, Chen H-L, Liu H-X, Xu D-M, Chen X, Huang X. Evaluation of the exposure risk of SARS-CoV-2 in different hospital environment. Sustainable Cities and Society. 2020;61:102413. doi: 10.1016/j.scs.2020.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M, Mohebbi SR, Hopke PK, Shahsavani A, Bazazpour S, Alipour MR, Jafari AJ, Bandpey AM, Zali A, Yarahmadi M, Farhadi M, Rahmatinia M, Hasanzadeh V, Nazari SSH, Asadzadeh-Aghdaei H, Tanhaei M, Zali MR, Kermani M, Vaziri MH, Chobineh H. Presence of SARS-CoV-2 in the air of public places and transportation. Atmospheric Pollution Research. 2021;12(3):302–306. doi: 10.1016/j.apr.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemati S, Mobini GR, Heidari M, Rahmani F, Babadi AS, Farhadkhani M, Nourmoradi H, Raeisi A, Ahmadi A, Khodabakhshi A, Sadeghi M, Bagheri M, Validi M, Taghipour S, Mohammadi-Moghadam F. Simultaneous monitoring of SARS-CoV-2, bacteria, and fungi in indoor air of hospital: a study on Hajar Hospital in Shahrekord, Iran. Environ Sci Pollut Res. 2021;10:1–11. doi: 10.1007/s11356-021-13628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lei C, Chen Z, Liu W, Hu X, Pei R, Hu J, Lei C, Chen Z, Liu W, Hu X, Pei R, Su Z, Deng F, Huang Y, Sun X, Cao J, Guan W. Distribution of airborne SARS-CoV-2 and possible aerosol transmission in Wuhan hospitals, China. National Science Review. 2020;7:1865–1867. doi: 10.1093/nsr/nwaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Li J, Yang J, Li J, Hong F, Long H, Deng Q, Qin Y, Jiang J, Zhou X, Song Q, Pan C, Luo P. SARS-CoV-2 presented in the air of an intensive care unit (ICU) Sustainable Cities and Society. 2020;65:102446. doi: 10.1016/j.scs.2020.102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayalar O, Arı A, Babuççu G, Konyalılar N, Doğan O, Can F, Şahin UA, Gaga EO, Kuzu SL, Arı PE, Odabaşı M, Taşdemir Y, Cindoruk SS, Esen F, Sakın E, Çalışkan B, Tecer LH, Fıçıcı M, Altın A et al (2021) Existence of SARS-CoV-2 RNA on ambient particulate matter samples: a nationwide study in Turkey. Sci. Total Environ. 789:147976 [DOI] [PMC free article] [PubMed]
- Lednicky JA, Lauzardo M, Fan ZH, Jutla A, Tilly TB, Gangwar M, Usmani M, Shankar SN, Mohamed K, Eiguren-Fernandez A, Stephenson CJ, Alam MM, Elbadry MA, Loeb JC, Subramaniam K, Waltzek TB, Cherabuddi K, Morris JG, Jr, Wu CY. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. International Journal of Infectious Diseases. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Helleis F, Borrmann S, Cheng Y, Drewnick F, Haug G, Klimach T, Sciare J, Su H, Pöschl U. Model calculations of aerosol transmission and infection risk of COVID-19 in indoor environments. International Journal of Environmental Research and Public Health. 2020;17:8114. doi: 10.3390/ijerph17218114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linillos-Pradillo B, Rancan L, Ramiro ED, Vara E, Artíñano B, Arias J. Determination of SARS-CoV-2 RNA in different particulate matter size fractions of outdoor air samples in Madrid during the lockdown. Environ. Res. 2021;195:110863. doi: 10.1016/j.envres.2021.110863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Martano P. Droplet fate in a cough puff. Atmosphere. 2020;11(8):841. doi: 10.3390/atmos11080841. [DOI] [Google Scholar]
- Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, Chao CYH, Li Y, Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T, Pintó RM, Bosch A, Moreno N, Alastuey A, Minguillón MC, Anfruns-Estrada E, Guix S, Fuentes C, Buonanno G, Stabile L, Morawska L, Querol X. Tracing surface and airborne SARS-CoV-2 RNA inside public buses and subway trains. Environment International. 2021;147:106326. doi: 10.1016/j.envint.2020.106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SWX, Tan YK, Chia PK, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M, Lednicky JA, Wu C-Y. Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology. 2019;127:1596. doi: 10.1111/jam.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos RG, Silveira MB, Abrahão JS. Exploratory assessment of the occurrence of SARS-CoV-2 in aerosols in hospital facilities and public spaces of a metropolitan center in Brazil. Environmental Research. 2021;195:110808. doi: 10.1016/j.envres.2021.110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintó RM, Costafreda MI, Bosch A. Risk assessment in shellfish-borne outbreaks of hepatitis A. Applied Environmental Microbiology. 2009;75(23):7350–7355. doi: 10.1128/AEM.01177-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivato A, Amoruso I, Formenton G, Di Maria F, Bonato T, Vanin S, Marion A, Baldovin T. Evaluating the presence of SARS-CoV-2 RNA in the particulate matters during the peak of COVID-19 in Padua, northern Italy. Science of The Total Environment. 2021;784:147129. doi: 10.1016/j.scitotenv.2021.147129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-COV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Ram K, Thakur RC, Singh DK, Kawamura K, Shimouchi A, Sekine Y, Nishimura H, Singh SK, Pavuluri CM, Singh RS, Tripathi SN. Why airborne transmission hasn’t been conclusive in case of COVID-19? An atmospheric science perspective. Science of the Total Environment. 2021;773:145525. doi: 10.1016/j.scitotenv.2021.145525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K, Castrica M, Menchetti L, Maggi L, Negroni L, Orfeo NV, Pizzoccheri A, Stocco MS, Balzaretti CM. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Science of the Total Environment. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotto A, Quaglino P, Lembo D, Morello M, Brizio E, Bardi L, Civra A. SARS-CoV-2 and indoor/outdoor air samples: a methodological approach to have consistent and comparable results. Environmental Research. 2021;195:110847. doi: 10.1016/j.envres.2021.110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia JL, Rivera DN, Herrera VL, Morwitzer MJ, Creager HM, Santarpia GW, Crown KK, Brett-Major DM, Schnaubelt ER, Broadhurst MJ, Lawler JV, Reid StP, Lowe JJ (2020). Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 29, 10(1):12732. [DOI] [PMC free article] [PubMed]
- Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Fontana F, Clemente L, Pallavicini A, Ruscio M, Piscitelli P, Miani A. SARS-CoV-2 RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environmental Research. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Koutrakis P, Martins MAG, Lemos B, Down SE, Sunderland EM, Garshick E. Characterization of hospital airborne SARS-CoV-2. Respiratory Research. 2021;22:73. doi: 10.1186/s12931-021-01637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo T, Liu X, Feng J, Guo M, Hu M, Guo D, Ullah H, Yang Y, Zhang Q, Wang X, Sajid M, Huang Z, Deng L, Chen T, Liu F, Xu K, Liu Y, Zhang Q, Liu Y et al (2020) ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerging Microbes & Infections 9(1):1259–1268 [DOI] [PMC free article] [PubMed]
- Tang JW, Bahnfleth WP, Bluyssen PM, Buonanno G, Jimenez JL, Kurnitski J, Li Y, Miller S, Sekhar C, Morawska L, Marr LC, Melikov AK, Nazaroff WW, Nielsen PV, Tellier R, Wargocki PI, Dancer SJ. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Journal of Hospital Infection. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosoughi M, Karami C, Dargahi A, Jeddi F, Jalali KM, Hadisi A, Haghighi SB, Dogahe HP, Noorimotlagh Z, Mirzaee AS (2021) Investigation of SARS-CoV-2 in hospital indoor air of COVID-19 patients’ ward with impinger method. Environ Sci Pollut Res. 10.1007/s11356-021-14260-3 [DOI] [PMC free article] [PubMed]
- Xu, I., Cai, J., Li, S., He, Q., Zhu, S., 2021. Airborne infection risks of SARS-CoV-2 in U.S. Schools and impacts of different intervention strategies. Sustainable Cities and Society, 103188. [DOI] [PMC free article] [PubMed]
- Yun H, Yang J, Seo JH, Sohn JR (2020) Methodology for sampling and detection of airborne coronavirus including SARS-CoV-2. Indoor and Built Environment. 10.1177/1420326X20980160
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4644 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.