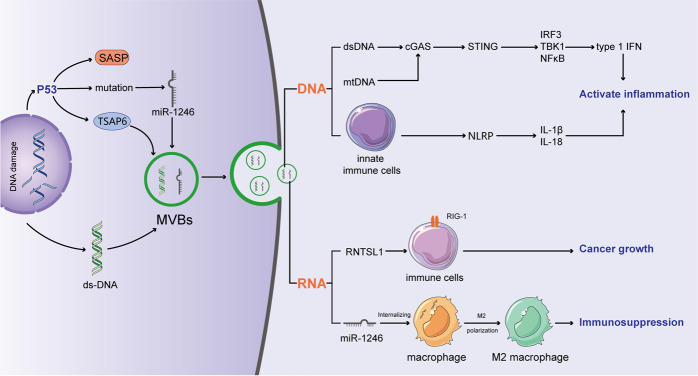
Fig. 3. DNA damage stress induced the release of immunomodulatory cargoes through EVs.
In DDR, P53 activated the tumor suppressor-activated pathway 6 (TSAP6) to increase the secretion of exosomes. Additionally, mutant P53 can selectively increase the release of miR-1246-enriched exosomes. And these miR-1246-enriched exosomes contribute to anti-inflammatory immunosuppression by inducing high levels of TGF-β activity. As a stress reaction, senescence enhanced the biosynthesis of exosome-like vesicles, and further several highly reactive agents were liberated via EVs. When cellular DNA damages appeared, EVs secretion contributed to maintain intercellular homeostasis via excreting the harmful cytoplasmic DNA fragments. dsDNA and mtDNA can upregulate the expressions of type I IFNs by activating cGAS/STING pathway. Additionally, exoDNA can be absorbed by innate immune cells in the intestine to stimulate the activation of the inflammasome and then enhance the secretion of IL-18 and IL-1β. RN7SL1 activates RIG-I in immune cells to accelerate the tumor growth. Importantly, dsRNA is deemed as a contributor of anti-cancer immunity since it recruits T cells into the tumor microenvironment through increasing the secretion of TLR3-dependent cytokines including type I IFN and CXCL10.