Figure 11.
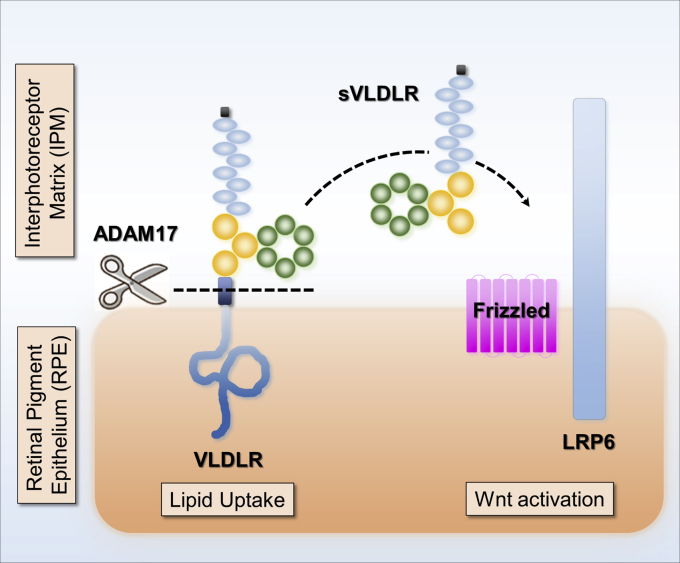
ADAM17-mediated VLDLR shedding modulates Wnt signaling in the eyes. Our previous studies have shown that VLDLR sheds its soluble extracellular domain, sVLDLR, to function as an endogenous inhibitor of canonical Wnt signaling by binding with Wnt receptor, LRP6. However, the sheddase that regulates VLDLR shedding remains unknown. In this study, we found that ADAM17 is the sheddase responsible for VLDLR shedding. ADAM17 liberates sVLDLR and enables its inhibitory effect on Wnt signaling. Meanwhile, ADAM17-mediated VLDLR shedding reduces the lipid uptake through full-length VLDLR. We found that ADAM17 cleaves both variant I and variant II of VLDLR. Future investigation is warranted to study the fate of remaining C terminus of VLDLR. ADAM17, a disintegrin and metalloprotease 17; LRP6, low-density lipoprotein receptor–related protein 6; sVLDLR, soluble ectodomain of VLDLR; VLDLR, very low-density lipoprotein receptor.