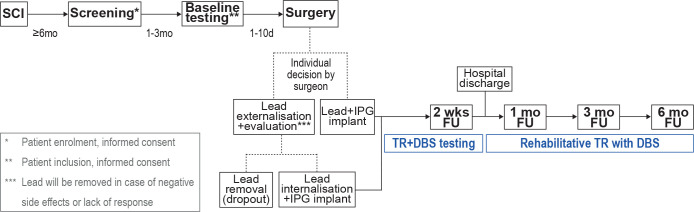
Figure 3.
Study timeline. Patients with a motor incomplete SCI at the level of T10 or above and at least 6 months of recovery after injury are eligible to undergo screening for study participation. Incomplete SCI is confirmed based on clinical examinations, MRI and electrophysiological measurements. One to three months after study enrolment, baseline testing is performed, followed by unilateral electrode implantation at the less severely affected side 1–10 days later. During surgery, the surgeon decides whether lead and impulse generator (IPG) will be implanted during one session, or whether the lead will be temporarily externalised, depending on intraoperative testing results. In case of lead externalisation, an evaluation period ensues where the patient’s responsiveness to MLR-DBS and potential negative side effects are assessed. In case of unsatisfactory results or withdrawal of consent, the lead is removed, and the patient is registered as a study dropout. In case of satisfactory testing, the lead is internalised and the IPG is implanted. After complete implantation, follow-up testing ensues at 2 weeks, 1 month, 3 months, and 6 months, respectively. Patients will be discharged from hospital after 2–3 weeks of training and testing. After hospital discharge, patients will undergo rehabilitative training with DBS at settings predefined during the first 2 weeks after implantation. d, day(s); DBS, deep brain stimulation; FU, follow-up; mo, month(s); SCI, spinal cord injury; TR, training; wks, weeks.