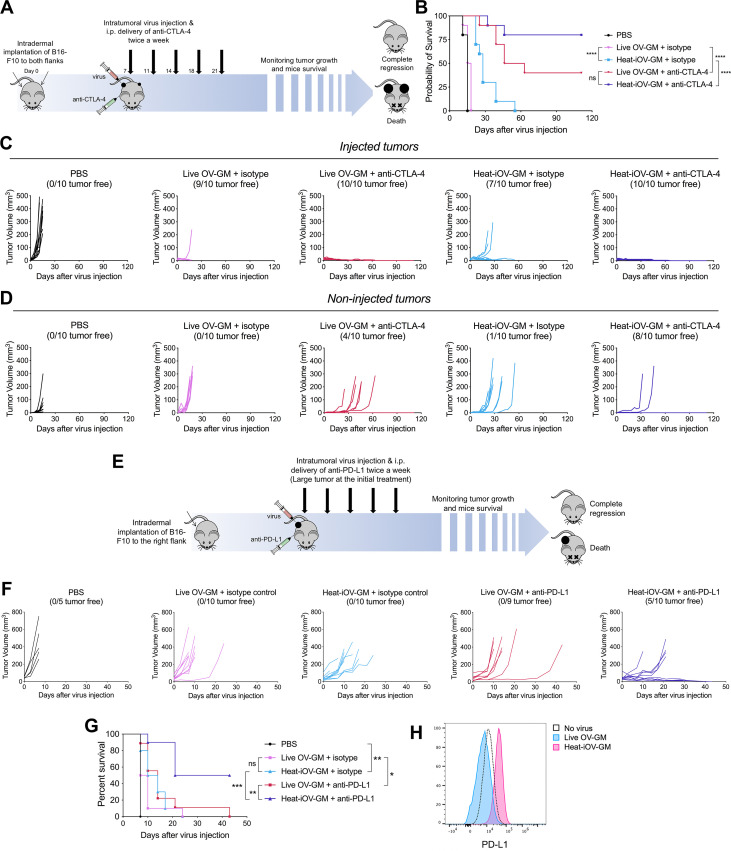
Figure 5.
Combination with immune checkpoint blockade further enhances the anti-tumor effects induced by heat-iOV-GM on non-injected tumors and large established tumors. (A) WT C57BL/6J mice were intradermally implanted with B16-F10 tumors into their left and right flanks. Starting from day 7 post-implantation, tumor-bearing mice were treated twice weekly with IT injection of live OV-GM or heat-iOV-GM in the combination of i.p. delivery of isotype control or anti-CTLA-4 antibody (n=10 for all groups). PBS was used as a control. Tumor volumes and mouse survival was monitored throughout the course of study. (B) Kaplan-Meier survival curves of WT mice treated with PBS, live OV-GM, or heat-iOV-GM with or without anti-CTLA-4 antibody (*P < 0.05; ***P < 0.001; ****P < 0.0001). (C) Tumor volumes of injected tumors over days of treatment. (D) Tumor volumes of non-injected tumors over days of treatment. (E) WT C57BL/6J mice were intradermally implanted with B16-F10 melanoma cells in their right flanks. When tumor diameters reached 5 mm, intratumoral injection of live OV-GM, or heat-iOV-GM combined with i.p. delivery of anti-PD-L1 or isotype control was initiated and continued twice a week. PBS was used as a control. Tumor growth and mouse survival were monitored throughout the course of the study. (F) Tumor volumes of injected tumors over days of treatment. (G) Kaplan-Meier survival curve of WT mice treated with PBS, live OV-GM or heat-iOV-GM with or without anti-PD-L1 (n=9 or 10; *P < 0.05; ***P < 0.001; ****P < 0.0001). (H) PD-L1 expression on B16-F10 cells infected by live OV-GM or heat-iOV-GM was measured by flow cytometry. Cells were infected at an MOI of 10 for 24 hours before staining.