Figure 4.
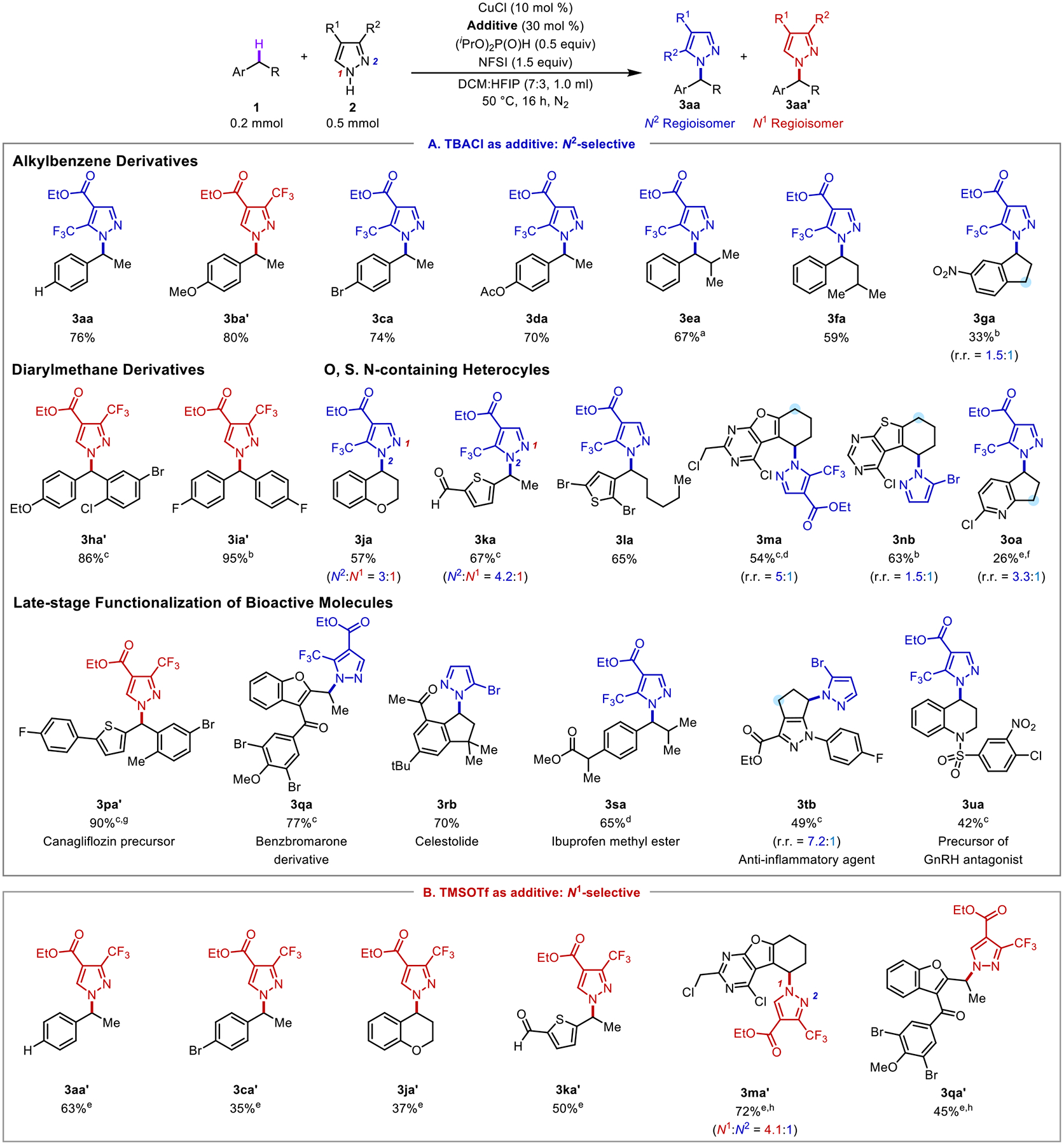
Assessment of various benzylic C–H substrates in cross coupling reactions with N–H heterocycles with (A) TBACl as the additive for N2 regioselectivity and (B) with TMSOTf as the additive for N1 regioselectivity. Regioisomers >5% were isolated and reported. aConducted in 0.5 mL DCM:HFIP (7:3). bConducted with 10 mol % CuBr2 and 30 mol % TBABr. c Conducted with 10 mol % TBACl. dConducted at 40 °C. eConducted at 60 °C. fConducted in DCM. gConducted at 30 °C. hConducted with 10 mol % BF3•OEt2.
