Figure 5.
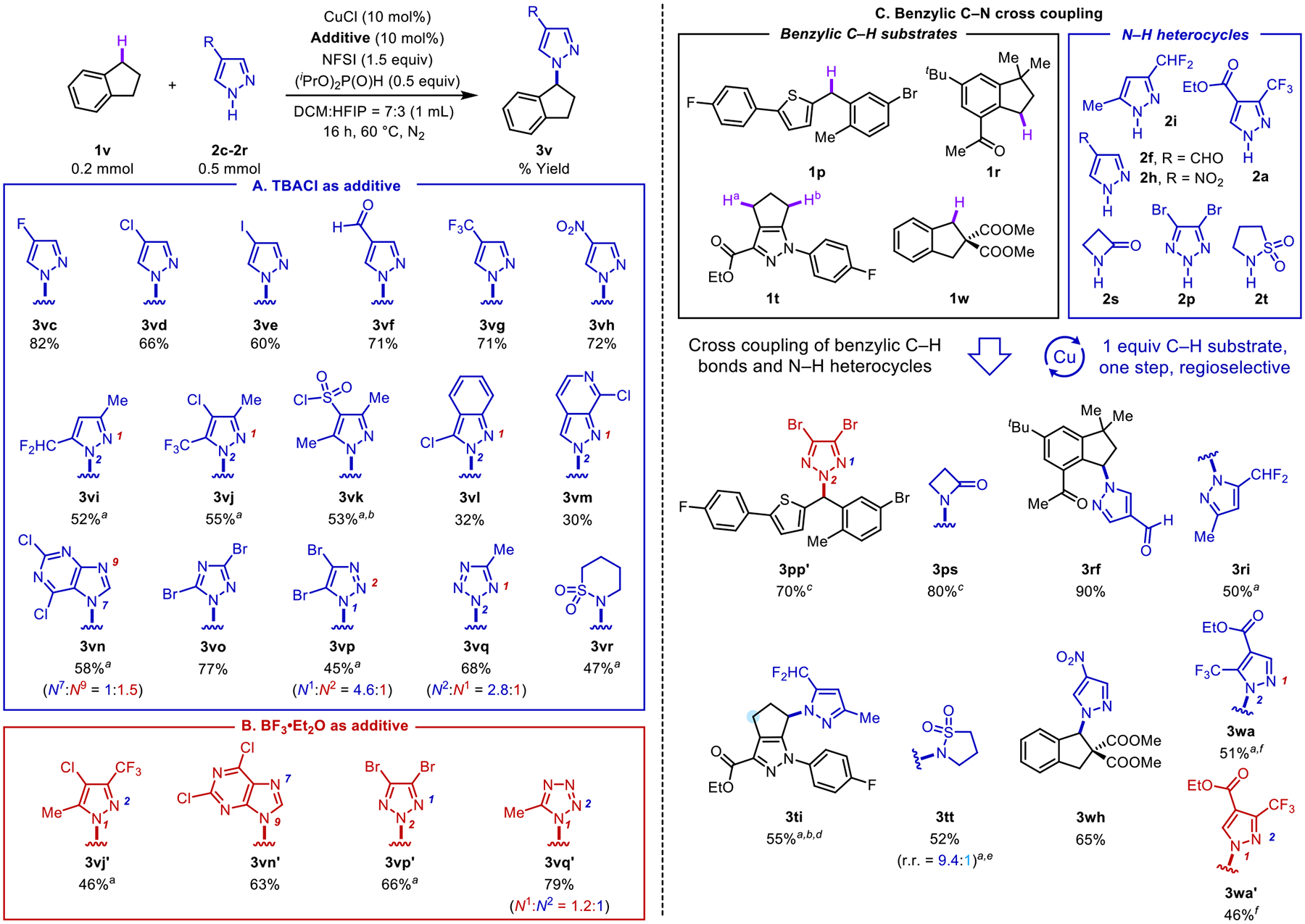
Assessment of various N–H heterocycles in cross coupling reactions. Substrate scope with diverse N–H heterocycles and indane under kinetically controlled TBACl conditions (A) and in the presence of BF3•OEt2 as a Lewis acid cocatalyst (B). Exploration of cross-coupling reactions of diverse N–H heterocycles and (hetero)benzylic C–H scaffolds (C) under the TBACl conditions of Figure 5A, unless noted otherwise. Regioisomers formed in >5% yield were isolated. aConducted in DCM. bConducted at 50 °C. cConducted at 30 °C. dTrace amount of the other benzylic regioisomer was observed. eConducted at 40 °C. fConducted with 10 mol % BF3•OEt2.
