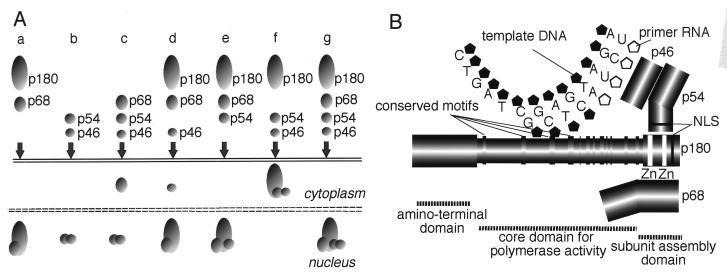
FIG. 8.
Schematic representations of the results of the present study. (A) Model for the nuclear transport pathway of mouse DNA polymerase α-primase complex which shows the subcellular distribution of two, three, or four of the coexpressed subunits of DNA polymerase α. (B) Organization of the subunit assembly of DNA polymerase α. The p180 molecule can be divided into three domains. The first is the amino-terminal domain (residues 1 to 329), which is dispensable for polymerase activity and subunit assembly. The second is the core domain (residues 330 to 1279), which is sufficient for DNA polymerase activities such as template recognition, substrate binding, and the phosphoryl transfer reaction. The third is the carboxyl-terminal domain (residues 1235 to 1465), which is dispensable for polymerase activity but required for assembly of the complex. p68 binds directly to the putative zinc finger motifs in the carboxyl-terminal domain, whereas p54-p46 associates with the carboxyl-terminal domain independently of the zinc finger motifs. The interaction between p46 and p180 is mediated by p54.