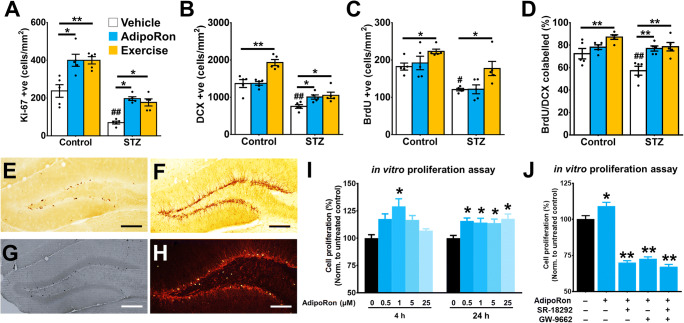
Fig. 2.
AdipoRon treatment mimicked the effect of physical exercise in restoring adult hippocampal neurogenesis in diabetic mice. A STZ-diabetes reduced number of Ki-67+ proliferating cell (Tukey’s post hoc test: ##P < 0.005 vs. Control-Vehicle). AdipoRon treatment (Tukey’s post hoc test: *P < 0.05 vs. Control-Vehicle; *P < 0.05 vs. STZ-Vehicle) mimicked the effect of exercise (Tukey’s post hoc test: **P < 0.005 vs. Control-Vehicle; *P < 0.05 vs. STZ-Vehicle) on increasing proliferating cell in both control and diabetic mice. n = 5 per group. Previously reported Control-Vehicle and Control-AdipoRon data were used to support this study (44). B STZ-diabetes reduced the density of DCX+ immature neuron (Tukey’s post hoc test: ##P < 0.005 vs. Control-Vehicle). AdipoRon treatment (Tukey’s post hoc test: **P < 0.005 vs. STZ-Vehicle) restored the density of immature neurons as running did in diabetic mice (Tukey’s post hoc test: **P < 0.005 vs. STZ-Vehicle), but not increasing the immature neuron density in control mice (Tukey’s post hoc test: *P > 0.05 vs. Control-Vehicle). Previously reported Control-Vehicle and Control-AdipoRon data were used to support this study (44). C STZ reduced cell survival in the DG (Tukey’s post hoc test: #P < 0.05 vs. Control-Vehicle) marked by BrdU. Voluntary running increased cell survival in both control (Tukey’s post hoc test: *P < 0.05 vs. Control-Vehicle) and diabetic animals (Tukey’s post hoc test: *P < 0.05 vs. STZ-Vehicle). However, AdipoRon treatment showed no significant effects (Tukey’s post hoc test: *P > 0.05 vs. STZ-Vehicle). Previously reported Control-Vehicle and Control-AdipoRon data were used to support this study (44). D STZ impaired neuronal differentiation (Tukey’s post hoc test: #P < 0.05 vs. Control-Vehicle) with reduced percentages of BrdU/DCX co-labelling. AdipoRon treatment (Tukey’s post hoc test: **P < 0.005 vs. STZ-Vehicle) restored neuronal differentiation of new-born cells in diabetic mice (Tukey’s post hoc test: #P < 0.05 vs. Control-Vehicle), as runners did. However, AdipoRon did not show effects on neuronal differentiation in healthy control mice (n = 5 animals per group). Previously reported Control-Vehicle and Control-AdipoRon data were used to support this study (44). E–H Representative image of E proliferating progenitor cell, marked by Ki-67, in the subgranular zone. F Representative image of immature neurons, marked by doublecortin (DCX). G New-born cell (3 days before treatment until day of sacrifice), marked by BrdU, in DG. H Representative image of BrdU/DCX co-labelled cells, representing the new-born cells committing the neuronal lineage. I In vitro study of the direct effect of AdipoRon on cell proliferation in culture N2a cells. AdipoRon exerted proliferating effect in a time- and dose-dependent manner (6 replicates per independent experiment; n = 3 independent experiments/treatment group) in CyQuant assay (LSD post hoc test: *P < 0.05 vs. Control). J In vitro examination of the involvement of PPAR-γ/PGC-1α signalling in AdipoRon-induced cell proliferation in culture N2a cells. AdipoRon-induced cell proliferation was mediated by PPAR-γ/PGC-1α signalling. Blocking either PPAR-γ by SR-18292 or PGC-1α by GW9662 or co-treatment diminished AdipoRon-induced cell proliferation (9 replicates per independent experiment; n = 4 independent experiments/group) (LSD post hoc test: *P < 0.05 vs. untreated control)