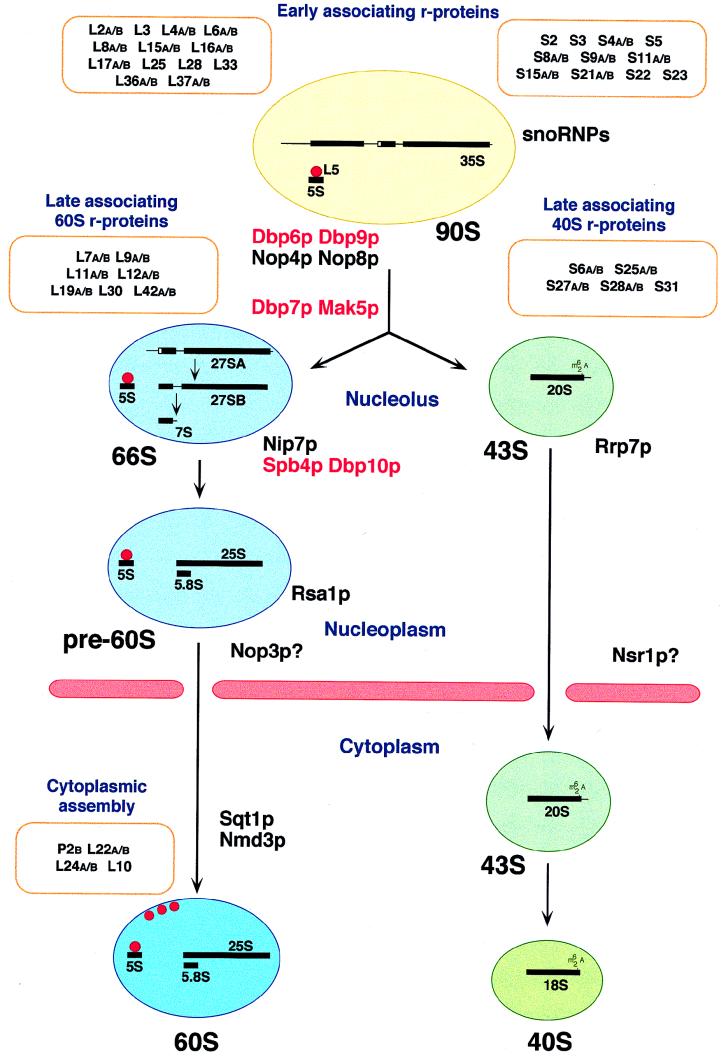
FIG. 2.
Ribosomal subunit assembly pathway in S. cerevisiae. In the nucleolus, the 35S pre-rRNA associates with many early-associating 40S and 60S r-proteins to form a 90S preribosome. The 5S rRNA forms a stable RNP with Rpl5p (L5, red dot). The 5S rRNA is probably already present in 90S preribosomes; therefore, Rpl5p may belong to the group of early-assembling 60S r-proteins. From the 90S particle, 66S and 43S preribosomes containing the 27S and 20S pre-rRNAs, respectively, are formed. The 66S and the 43S preribosomes contain the late-associating r-proteins. Preribosomes are most likely actively exported through nuclear pores. It has been demonstrated that export of 60S ribosomal subunits requires the nuclear or cytoplasmic Ran cycle and distinct nucleoporins (not shown; for details, see reference 70). It is believed that the 66S particle remains in the nucleus until the 7S pre-rRNA is processed to the mature 5.8S rRNA, while the 43S preribosome is rapidly exported to the cytoplasm, where the final maturation step in the synthesis of the 18S rRNA takes place. Assembly of 60S ribosomal subunits is also completed in the cytoplasm by the final incorporation of some r-proteins. At least three of them (also depicted as red dots), including the P2B and Rpl10p/Qsr1p (L10) r-proteins, can exchange on mature 60S ribosomal subunits in vivo. Preribosomes are drawn as balloons, and the nuclear envelope is depicted as pink rods. Protein trans-acting factors with likely involvement in the assembly of ribosomal subunits are colored as follows: red, putative ATP-dependent RNA helicases; black, others. Only those r-proteins for which an indication about the timing of their association with preribosomal particles is known are shown. See the text for further details.