Figure 4. BT-GSI decreases MM-induced osteolytic lesions and osteoclasts in immunocompetent mice with established MM.
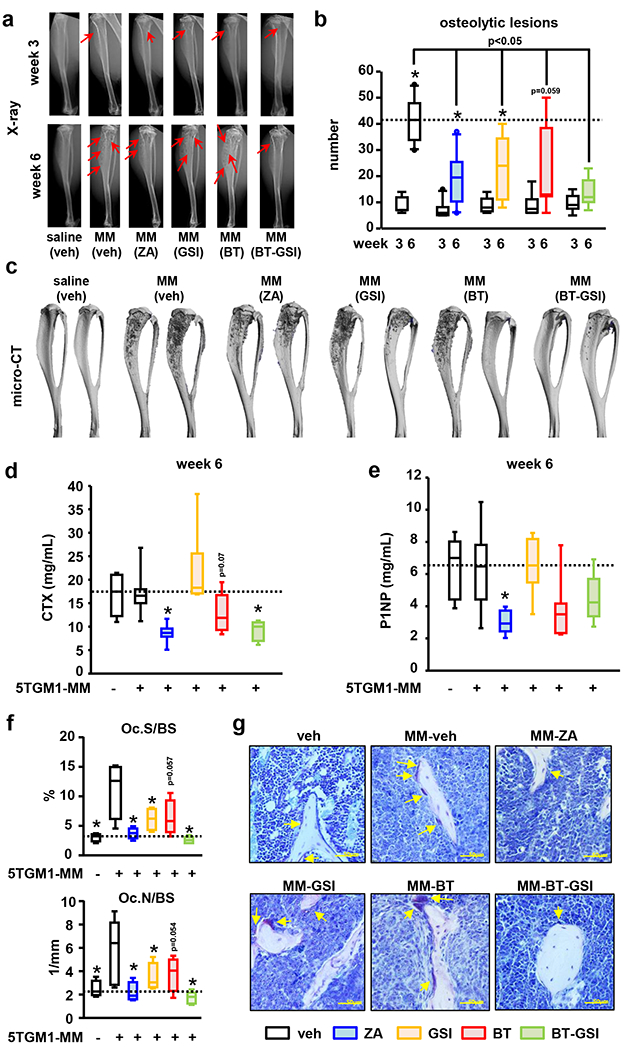
Effects of zoledronic acid (ZA, 0.1 mg/kg, two times a week , for 3 weeks) and equimolar dosing (0.1mol/L, three times a week, 3 weeks) of GSI, BT, and BT-GSI on (a-b) osteolytic lesion number per bone, (c) bone destruction (microCT 3D reconstruction), (d) serum levels of the bone resorption marker CTX (week 6), (e) the bone formation marker P1NP (week 6), and on (f) bone surface covered by osteoclasts (Oc.S/BS) and osteoclast number (Oc.N/BS). (g) Representative images of TRAP stained bone histological sections. Yellow arrows indicate TRAP+ osteoclasts. Bars represent means ± SD. n=6-11/group. *p<0.05 vs week 3 (b), vs MM (veh; d-e), or vs naïve mice (f). Red arrows indicate osteolytic lesions. Horizontal dotted lines indicate the mean value for vehicle-treated mice bearing MM tumors (b, d, e) or naïve mice (f).
