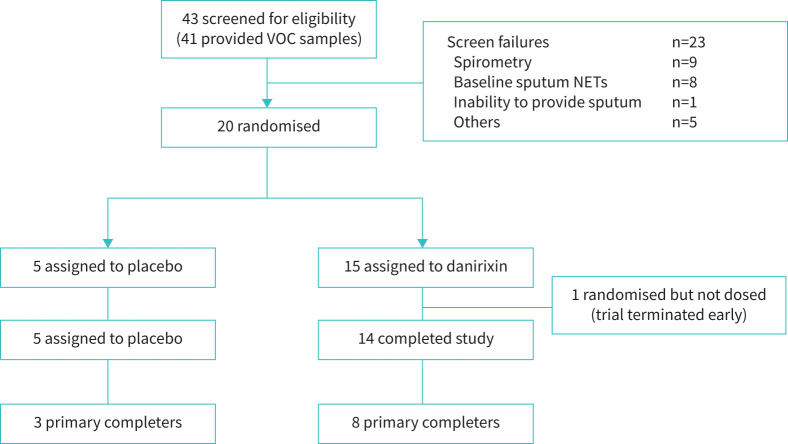
FIGURE 1.
Consolidated Standards of Reporting Trials diagram for trial participants. The “primary completer” population was defined via subjects who provided “good”- or “acceptable”-quality sputum samples (based on percentage of squamous cells and viable leukocytes) at baseline and day 14. The primary completer population was used for sputum neutrophil extracellular traps (NETs) analyses, but the entire study population was used for the microbiome analysis. VOC: volatile organic compound.