Figure 6. Proposed mechanism of ATP binding to SERCA.
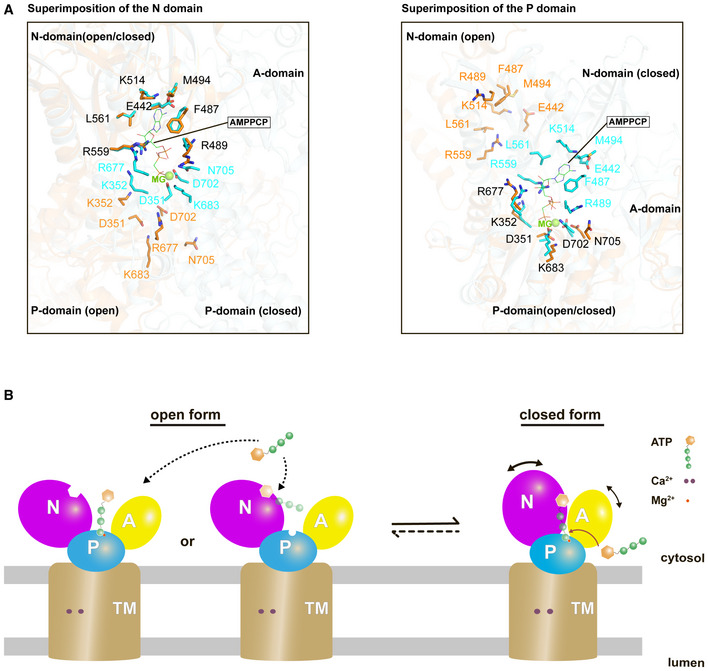
- Closed‐up views of the ATP binding residues when the N domain (left) or the P domain (right) is superimposed between the cryo‐EM structure of SERCA2b in the E1∙2Ca2+‐AMPPCP state (cyan; PDB ID: 6LLE) and the “open‐form” crystal structure of SERCA1a in the E1∙2Ca2+ state (orange; PDB ID: 1SU4). The ATP binding residues in the former and the latter are represented by cyan and orange sticks, respectively. A Mg2+ ion close to the AMPPCP molecule is depicted as a green sphere. Note that all residues are numbered according to the residue number in SERCA2b.
- The present cryo‐EM structure of SERCA2b in combination with the previous crystal structure of SERCA1a (PDB ID: 1SU4) reveals multiple conformations of SERCA in the E1∙2Ca2+ state due to the highly mobile cytosolic domains, which could be in equilibrium between closed and open forms. While the open form largely exposes the ATP‐binding site and therefore may readily receive and release ATP, the closed form appears to provide an ATP entry gate and serves as a preformed state immediately before ATP binding to SERCA.
