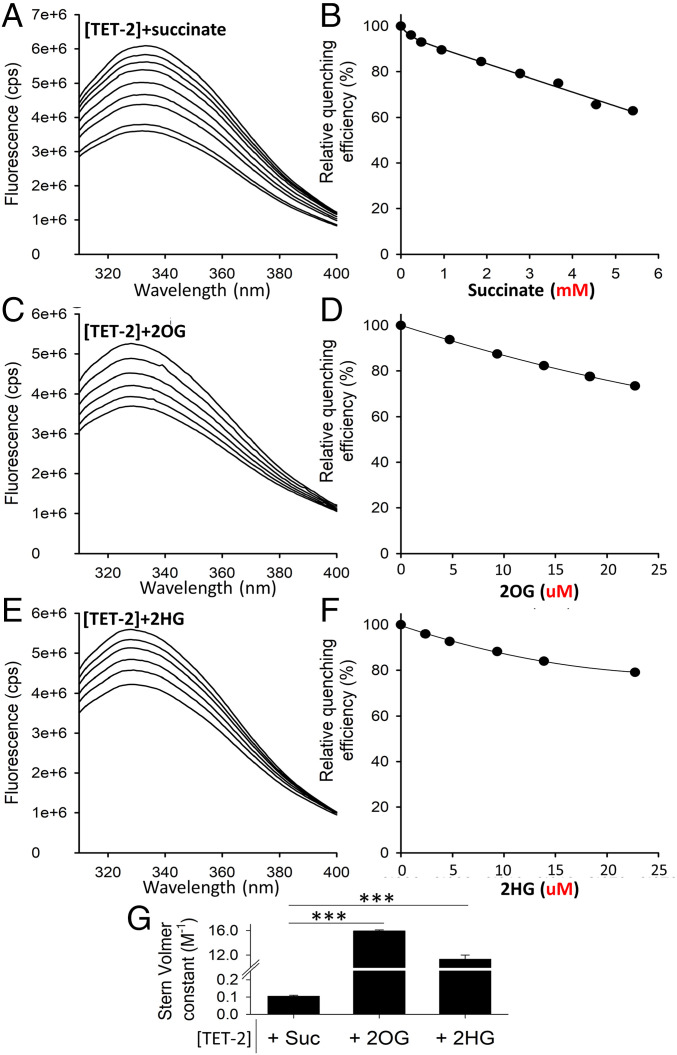
Fig. 7.
Succinate-induced decrease in TET-2 activity is likely via product inhibition (rather than competitive inhibition). Fluorescence quenching is a technique for measuring binding affinity between ligands and proteins. It is the decrease in quantum yield of fluorescence from a fluorophore, induced by molecular interactions with the quencher molecule(s). This experiment was undertaken to determine the binding affinity between succinate and the catalytic domain of recombinant human TET-2 protein (in comparison with the binding affinity of 2OG [natural cosubstrate] and 2HG [known competitive inhibitor] with the catalytic domain of TET-2). (A, C, E) Fluorescence spectra of 0.5 μM TET-2 are shown after excitation at 280 nm with increasing amounts of Succinate, 2OG, and 2HG (from top to bottom, respectively). (B, D, F) The relative fluorescence intensity at 328 nm is shown as a function of Succinate, 2OG, and 2HG. (Note the millimolar concentrations for succinate and micromolar concentrations for 2OG and 2HG in the relative quenching efficiency). (G) Comparison of the Stern-Volmer constants ± SEM obtained from the Stern-Volmer equation (Eq. 1 in Fluorescence Spectroscopy). Stern-Volmer constants ± SEM for [TET-2]+succinate, [TET-2]+2OG, and [TET-2]+2HG are 0.1 ± 0.0, 15.7 ± 0.1, and 12.2 ± 0.5, respectively. Put together, the binding affinity of succinate to the catalytic domain of TET-2 is less than a hundredth that of 2OG (natural cosubstrate) and 2HG (known competitive inhibitor), suggesting that the mechanism of succinate-induced inhibition of TET-2 is not via competitive inhibition but via product inhibition (each reaction of TET-2 utilizes 2OG as a cosubstrate and converts it to succinate).