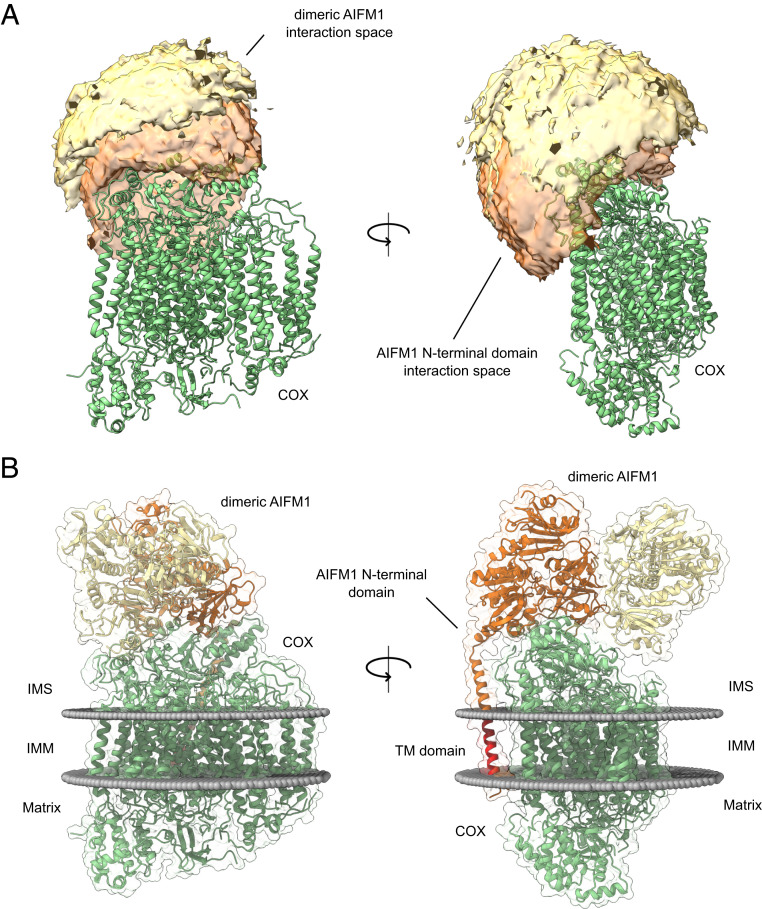
Fig. 3.
Cross-link–derived structural model of the COX-AIFM12 complex. (A) Visualization of the cross-link–driven accessible interaction space models for a COX-AIFM12 complex. COX is represented in green, while the bright orange volume represents the center-of-mass position of the AIFM1 dimer, and the dark orange volume represents the center-of-mass position of the model of the AIFM1 N terminus (residues 55 to 124). The cross-linking data are consistent with the interaction space available for docking dimeric AIFM1 and the N-terminal region of one AIFM1 protomer to monomeric COX. (B) Cross-link–derived structural model of the COX-AIFM12 complex. COX is represented in green, and AIFM1 protomers (residues 128 to 516; 551 to 613) with and without N-terminal region (residues 55 to 127) are represented in orange and yellow, respectively. The transmembrane residues (67 to 85) of the N terminus of the interacting AIFM1 moiety is highlighted in red. Membrane boundaries of the inner mitochondrial membrane are sketched as gray spheres. The final complex consists of monomeric COX, dimeric AIFM1 (residues 128 to 516, 551 to 613), and the N-terminal region of one AIFM1 protomer (residues 55 to 127).