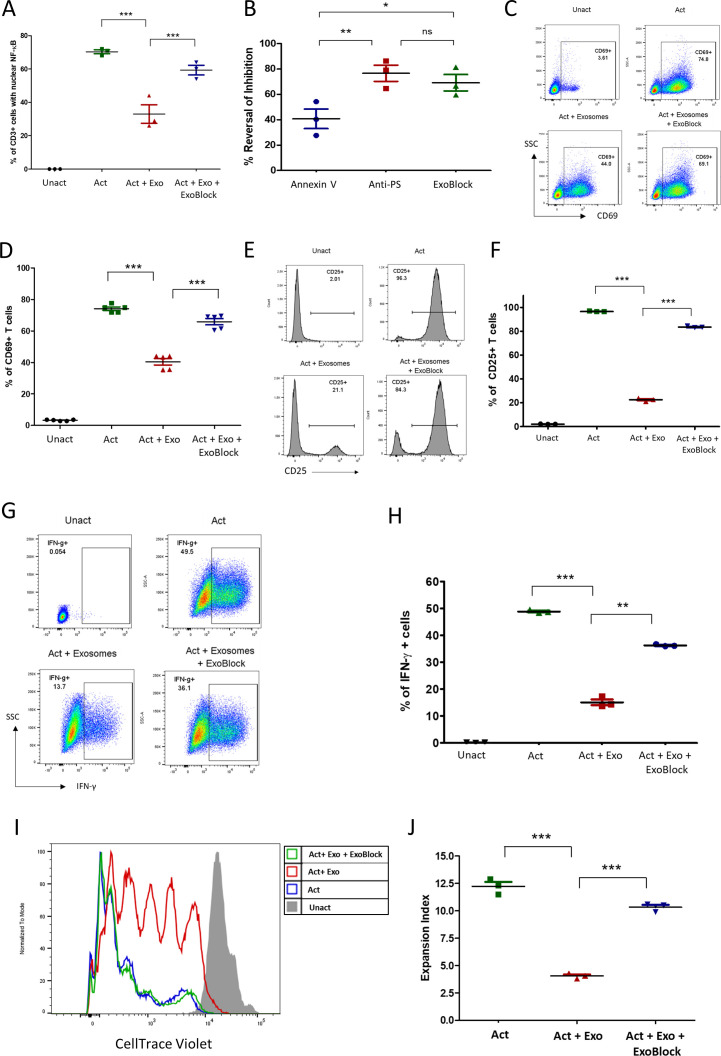
Figure 3.
ExoBlock reverses immunosuppression mediated by ovarian tumor-associated exosomes in vitro. (A–D) T cells were either left unactivated (Unact), activated for 2 hour with immobilized antibodies to CD3 and CD28 without exosomes (Act), with exosomes only (Act + Exo), or with exosomes and ExoBlock (Act + Exo + ExoBlock). Activation was monitored by detecting the nuclear translocation of NFκB using confocal microscopy (A, B) or by detecting the upregulation of CD69 on CD3+ T cells using flow cytometry following overnight incubation (representative data shown in C, quantified data shown in D). (B) A comparison of the in vitro efficacies of different PS-binding molecules in terms of reversing inhibition of NFκB translocation. (E–H) T cells were either left Unact, activated for 72hours with immobilized antibodies to CD3 and CD28 without exosomes (Act), Act + Exo, or Act + Exo + ExoBlock. Activation was monitored by detecting the upregulation of CD25 (representative data shown in E, quantified data shown in F) or the expression of intracellular IFN-γ (representative data shown in G, quantified data shown in H). (I–J) T cells labeled with CellTrace violet were either left Unact, activated for 7 days with immobilized antibodies to CD3 and CD28 without exosomes (Act), Act + Exo, or Act + Exo + ExoBlock. Proliferation was assessed by monitoring dye dilution using flow cytometry. (I) Representative dye dilution profiles and (J) expansion indices (depicting fold expansion of the culture) are shown. n=3. Data shown as mean±SEM. *P≤0.05, **P≤0.01, ***P≤0.001. Act + Exo, Act with exosomes only; Act + Exo + ExoBlock, Act with exosomes and ExoBlock;SSC, side scatter; IFN-γ, interferon gamma; ns, not significant; PS, phosphatidylserine; Unact, unactivated.