Abstract
Some studies have assessed the expression of dopaminergic dopamine 2 (D2)/3 receptors in prolactinomas and nonfunctioning pituitary adenomas (NFPA) by positron emission tomography/computed tomography (PET/CT) with 11C-raclopride, proving that this modality can be useful to predict the response to treatment with dopamine agonists. However, the use of 11C-labeled radiotracers is limited, as it requires a cyclotron in the PET center. 18F-fallypride is a radiotracer that has proven useful in assessing the expression of D2/3 receptors. As it is labeled with 18F, it can be produced and transported to distant PET centers. There are no studies on the usefulness of 18F-fallypride for the evaluation of patients with prolactinomas and NFPA. The aim of this study was to describe the first case series of patients with prolactinomas and NFPA studied with 18F-fallypride and 11C-methionine PET/CT to reveal D2/3 expression and amino acid (AA) metabolism. 18F-fallypride and 11C-methionine uptake were assessed in a case series of six patients, five with prolactinomas and one with a NFPA, and compared with clinical presentation and follow-up at 6–18 months. All patients presented with macroadenomas, with a wide range of AA metabolism, as revealed by 11C-methionine PET/CT. 18F-fallypride PET/CT identified low to moderate/high D2/3 expression in the tumors. The patient that presented low expression of D2/3 in the tumor and high AA metabolism showed a poor response to DA therapy. 18F-fallypride was able to reveal D2/3 receptor expression in prolactinomas and NFPA, with the advantage of been a more accessible radiotracer in comparison with previous 11C labeled analogs.
Keywords: 11C-methionine, 18F-fallypride, nonfunctioning adenoma, positron emission tomography, prolactinoma
INTRODUCTION
Prolactinomas are the most frequent type of pituitary tumors, with a prevalence of 50 per 100,000 inhabitants.[1] They account for approximately 50% of pituitary adenomas.[1] Currently, the majority of prolactinomas are treated with dopamine agonists (DAs), which can be used either to treat primary tumors or recurrences.[1] Although the majority of patients respond adequately to low doses of DAs, some patients may require high doses or be resistant to drug treatment.[1]
Nuclear neuroimaging with tracers, such as 11C-raclopride, 123I-epidepride, or 123I-IBZM, for dopamine 2 (D2) receptors have proven useful for the study of prolactinomas.[2,3,4,5] Tumors with high levels of D2 receptors, as evaluated by nuclear neuroimaging, respond better to treatment with DAs as compared with that of tumors with low levels of these receptors.[4,5] All these previous studies have established a proof of principle for the use of dopaminergic receptor tracers in the prognostic evaluation of prolactinomas.
Positron emission tomography/computed tomography (PET/CT) provides images of cellular and molecular events, with higher spatial resolution in comparison to single-photon emission CT systems. A number of studies have demonstrated the accuracy of 11C-methionine PET/CT for the detection of pituitary adenomas and evaluation of recurrence.[6,7,8,9] Thus far, only two PET tracers have been used in patients to determine D2 receptor expression in prolactinomas: 11C-raclopride and 11C-N-methylspiperone.[4,10,11] These tracers are unsuitable for extended clinical use because they are labeled with a short-life radionuclide (11C: 20 min half-life). Therefore, they can only be used in centers with onsite cyclotrons. This has led to limited use of PET/CT dopamine receptor imaging for the study of prolactinomas and nonfunctioning adenomas.
The performance of the radiotracer 18F-fallypride in evaluating the expression of D2/3 receptors in normal controls and patients with movement disorders and psychiatric conditions has being well-characterized.[12,13,14] As it is labeled with 18F (half-life of 110 min), it can be synthesized in a center with an onsite cyclotron and then transported for use in distant clinics. Although a previous study described the use of 18F-fallypride in animal models of prolactinomas,[15] there are no published papers on the use of this tracer in patients with prolactinomas and nonfunctioning adenomas.
The objective of this paper is to report our initial clinical experience in patients with prolactinomas and nonfunctioning adenomas studied with 18F-fallypride and 11C-methionine PET/CT.
MATERIALS AND METHODS
All patients provided signed written informed consent before inclusion in the study. The study group comprised a case series of six patients (age range: 27–69 years; females, n = 3), all of whom were referred to our center with a diagnosis of a prolactinoma (n = 5) or a nonfunctioning pituitary adenoma (n = 1). All six patients underwent PET/CT with both 18F-fallypride and 11C-methionine within 1 mo. Table 1 provides a summary of relevant clinical data. Follow-up took place 6–18 mo after the PET/CT studies.
Table 1.
Summary of clinical data, positron emission tomography/computed tomography findings and follow-up
| Patient | Age (years) | Sex | Presentation | Serum prolactin (ng/mL) | MRI findings | 18F-fallypride tumour uptake (percentage relative to the putamen) | 18F-fallypride tumour uptake (relative to the cerebellum) | 11C-methionine (HS/normal uptake in the cerebellum) | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | Male | Prolactinoma | 1006 | Macroprolactinoma 26 × 20 × 20 mm | 31 | 3,8 | 1,1 | Treatment with cabergoline. Reduction of serum prolactin to 16.0 ng/mL, with no change in tumor size |
| 2 | 63 | Female | NFPA | 35.3 | Macroadenoma 44 × 25 × 23 mm. Extra-pituitary extension | 120 | 15,4 | 4,1 | The patient had previous surgery and radiotherapy. Cabergoline was initiated at 2 mg/week. The lesion remained stable on MRI |
| 3 | 27 | Female | Prolactinoma | 470 | Cystic macroprolactinoma 34 × 15 × 14 mm | 30 | 5,6 | 1,4 | Treatment with cabergoline. Reduction in tumor size |
| 4 | 64 | Male | Prolactinoma | 1447 | Cystic macroprolactinoma 18 × 15 × 15 mm | 18 | 2,3 | 1,6 | Treatment with cabergoline. Normalization of serum prolactin, with no change in tumor size on MRI |
| 5 | 42 | Male | Prolactinoma | 356 | Cystic macroprolactinoma 27 × 30 × 20 mm | 70 | 11,5 | 3 | Treatment with cabergoline. reduction in tumor size |
| 6 | 46 | Female | Prolactinoma | 270 | Macroprolactinoma 20 × 20 × 18 mm. Extra-pituitary extension | 12 | 4,1 | 3,8 | The patient underwent surgery due to a poor response to cabergoline |
Clinical data, 18F-fallypride and 11C-methionine uptake and follow-up. NFPA: Nonfunctioning pituitary adenoma; HS: Hotspot; MRI: Magnetic resonance imaging
For 11C-methionine PET/CT, the patients were comfortably positioned in a Discovery PET/CT 690 camera (GE HealthCare) and low-dose CT was performed with the following protocol: 140 kV, 120 mA, rotation time 0.8 s, slice thickness 3.75 mm, Pitch 0.984, speed 39.73. For the emission study, an intravenous activity of approximately 5 MBq/kg of 11C-methionine was administered and an acquisition of 3D frame PET during 35 min was started (1 min, 2 min, 3 min, 9 min, and 20 min). The images obtained were reconstructed using ordered subset expectation maximization (OSEM) VUEPOINT. The parameters were as follows: 2/24 iterations/subsets; matrix: 128 × 128 pixels; Z axis filter: Standard post filter; 2, 1 diameter 25.
For the 18F-fallypride study, the patients were positioned in the same camera and a CT scan of the head was performed with the following protocol: 140 kV, 120 mA, rotation time 0.8 s, slice thickness 3.75 mm, Pitch 0.984, and speed 39.73. A dose of approximately 3 MBq/kg of 18F-fallypride was then administered intravenously, and acquisition of the 3D images (24 frames: 4 × 30 s, 9 × 60, 3 × 180 s and 8 × 300 s) was started. If the patients could not tolerate the full dynamic acquisition, a 4 frame × 300 s scan starting at 40 min post injection was performed. The images were reconstructed using OSEM VUEPOINT (2/24 iterations/subsets, 128 × 128-pixel matrix, post filter: 3.3 diameter 25).
Image analysis
The images were analyzed using the software PMOD (version 3.8, PMOD Technologies Ltd., Zurich, Switzerland). For the 11C-methionine scans, a region of interest (ROI), which included four pixels of the highest uptake spot in the tumor, was drawn and compared with the mean uptake in a ROI in the cerebellum (hotspot/cerebellum index), at 15–35 min post injection as described by Bergström et al.[4] Methionine uptake was considered mild when tumor/cerebellum ratio was <1.4, moderate when 1.4–2, and high when >2.
For 18F-fallypride, the uptake in the spot with the highest activity (hotspot) was compared with the uptake in a normal putamen (reference tissue with high D2/3 receptor expression) and with the mean activity in the ROI in the cerebellum at 55–60 min post injection (time at which pituitary/cerebellum activity reaches an equilibrium as described by Mukherjee et al.).[13] This time frame allow us to compare patient tumor/cerebellum ratio with previously reported pituitary/cerebellum ratio in normal controls[13](see Discussion). 18F-fallypride uptake was considered mild when <30% of the putamen, moderate when 30%–69%, and high when ≥70% of the putamen.
RESULTS
The patients' serum prolactin levels ranged from 35.3 to 1447.0 ng/mL with five of the six patients having values higher than 270. All had macroadenomas, defined as tumors larger than 10 mm, with maximum diameters ranging from 18 to 44 mm. Three patients had a cystic component on magnetic resonance imaging (MRI), and two patients had extra-pituitary extension. Patient 6 presented with extension of the lesion to the sphenoidal and cavernous sinus and to the suprasellar cisterna, and patient 2 presented with compression of the optic chiasm.
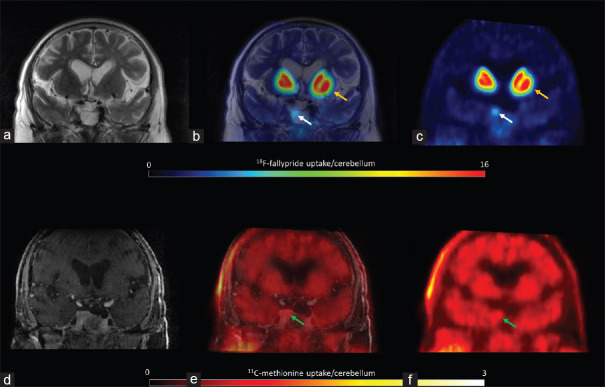
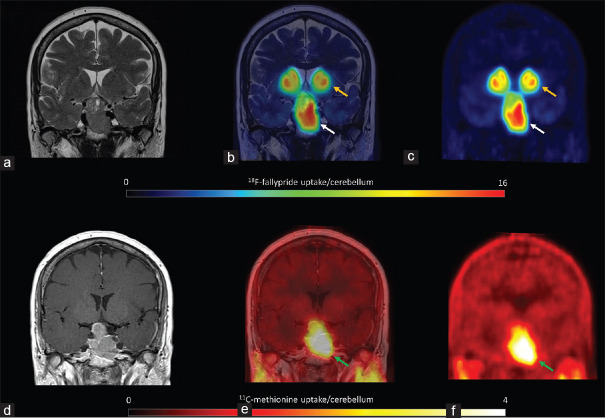
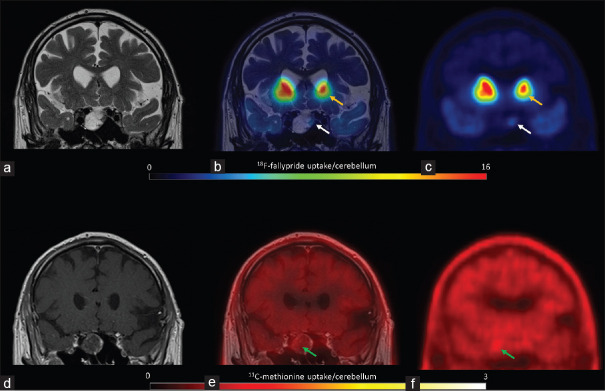
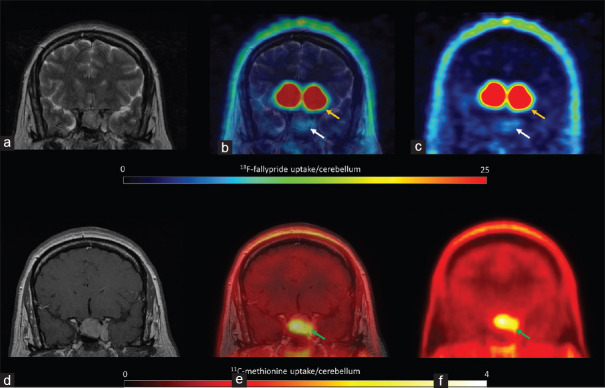
11C-methionine uptake was positive in all patients [hotspot/cerebellum index range: 1.1–4.1, Table 1], revealing amino acid (AA) transport and metabolism. In three of the six patients, including the two patients with extra-pituitary extension, the hotspot/cerebellum index was more than 3, indicating aggressive disease. Figure 1 shows low 11C-methionine uptake and moderate D2/3 receptor expression (patient 1), and Figure 2 shows both high 11C-methionine uptake and high D2/3 receptor expression (patient 2).
Figure 1.
Patient 1 T2 magnetic resonance imaging coregistered with 18F-fallypride positron emission tomography showing moderate expression of D2/D3 receptors (a, b and c, white arrows), lower than the uptake in the putamen (yellow arrows). 11C-methionine positron emission tomography coregistered with contrast-enhanced T1 magnetic resonance imaging showed low amino acid metabolism (d, e and f, green arrows)
Figure 2.
Patient 2 18F-fallypride positron emission tomography coregistered with T2 magnetic resonance imaging showed high uptake (a, b and c, white arrows), similar to the uptake in the putamen (yellow arrows). 11C-methionine positron emission tomography coregistered with contrast-enhanced T1 magnetic resonance imaging showed high amino acid metabolism (d, e and f, green arrows)
18F-fallypride showed moderate to high D2/3 receptor expression in the tumors of four of the six patients [Figures 1-3 and 5], as compared with the uptake in the normal putamen (range: 30%–120%). Mild D2/3 receptor expression was detected in the tumors of two patients (patients 4 and 6) as compared with that in the putamen (18 and 12%, respectively), as shown in Figures 4 and 6. In all patients, the 18F-fallypride uptake index in relation to the cerebellum ranged from 2.3 to 15.4.
Figure 3.
Patient 3. T2 MRI coregistered with 18F-fallypride PET showed moderate uptake (a, b and c, white arrows), lower to the uptake in the putamen (yellow arrows). 11C-methionine PET coregistered with contrast-enhanced T1 MRI showed moderate amino acid metabolism (d, e and f, green arrows)
Figure 5.
Patient 5. T2 MRI coregistered with 18F-fallypride PET showed high uptake (a, b and c, white arrows), lower to the uptake in the putamen (yellow arrows). 11C-methionine PET coregistered with contrast-enhanced T1 MRI showed high amino acid metabolism (d, e and f, green arrows)
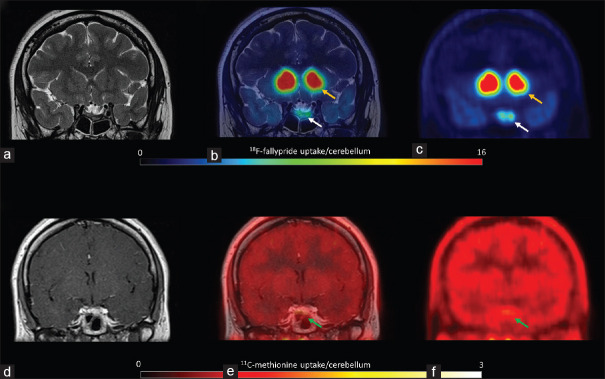
Figure 4.
Patient 4. 18F-fallypride PET coregistered with T2 MRI showed low uptake (a, b and c, white arrows) compared to the putamen (yellow arrows). 11C-methionine PET coregistered with contrast-enhanced T1 MRI showed moderate amino acid metabolism (d, e and f, green arrows)
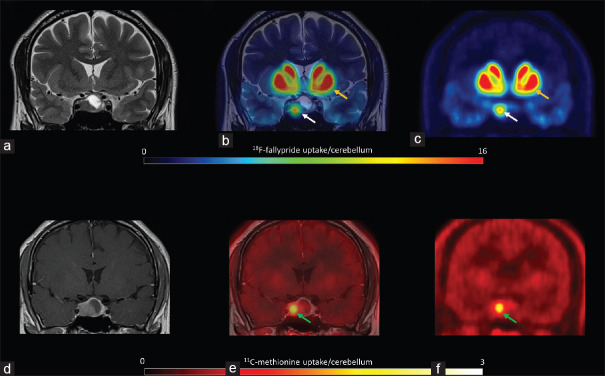
Figure 6.
Patient 6 18F-fallypride positron emission tomography coregistered with T2 magnetic resonance imaging showed low expression of D2/D3 receptors (a, b and c, white arrows) in comparison with that in the putamen (yellow arrows). The tumor showed high 11C-methionine uptake (d, e and f, green arrows)
The comparison of 11C-methionine and 18F-fallypride uptake showed that two of the patients with higher D2/D3 receptor expression (patients 2 and 5) also had a high 11C-methionine uptake index. In contrast, patient 6 had high 11C-methionine (hotspot/cerebellum index: 3.8) uptake but mild D2/3 receptor expression (12% compared to the putamen, 4.1 compared to the cerebellum) [Figure 6].
In the follow-up 6–18 mo later, all the patients were treated with cabergoline. In four of the six patients, there was either a reduction in serum prolactin levels or a reduction in tumor size [Table 1]. At follow-up, the nonfunctioning pituitary adenoma remained stable on MRI [Figure 2]. The patient with the lowest 18F-fallypride percentage uptake as compared with that in the putamen and high AA metabolism, as revealed by 11C-methionine, required surgery due to a poor response to cabergoline [Figure 6].
DISCUSSION
We presented the first case series of patients with prolactinomas and nonfunctioning adenomas studied with 18F-fallypride to reveal the expression of D2/3 dopamine receptors. We also investigated AA metabolism, as determined by 11C-methionine PET/CT.
The previous research on the expression of dopaminergic receptors in patients with prolactinomas showed that receptor expression could help to determine whether DA treatment would be effective.[5] Bergström et al. showed that D2 receptor expression, as revealed by 11C-raclopride, in patients with prolactinomas may establish the prerequisites for dopaminergic treatment.[16] Another study described the usefulness of the PET radiotracer 11C-N-methylspiperone in evaluating D2 receptor expression in prolactinomas.[10]
In addition to the advantage of its availability in comparison with other radiotracers, 18F-fallypride has shown greater affinity for D2/3 receptors and a better signal/noise ratio compared to 11C-raclopride.[17] It has also shown better performance than 11C-raclopride for the quantification of the extra striatal regions in which there is a lower density of receptors compared to the striatum. 18F-fallypride has proven to be the only radiotracer with good performance to assess the expression of D2/3 receptors in both the striatum and in extrastriatal regions. However, the cost of the study could be a limitation for its extended clinical use in patients with prolactinomas and nonfunctioning pituitary adenomas.[17,18]
Although the mechanism of prolactinoma resistance to DA treatment is not fully understood, the low expression of dopamine receptors may play a role.[19,20] In cases where medication fails to control the size and function, other options, including switching to another DA, increasing the DA dose, surgery, radiotherapy, and experimental treatments may be implemented.[1]
Several radiotracers have proved to be useful for the study of pituitary adenomas.[6]18F-FDG has shown a good sensitivity and specificity for the detection of macroadenomas[21] and functioning pituitary microadenomas.[22] The performance of 11C-methionine for the evaluation of AA metabolism in hypophyseal tumors has also been assessed previously.[16,23] Feng et al. reported that 11C-methionine was superior to 18F-FDG in the evaluation of patients with recurrent microadenomas.[23] Somatostatin receptor expression has been studied in patients with pituitary tumors using 68Ga-DOTATATE PET/CT. This modality proved useful to detect both functioning and nonfunctioning adenomas,[6,24,25,26] with better performance in the detection of normal pituitary tissue remaining after surgery in comparison with 18F-FDG, that may be more useful to detect the residual tumor.[27]
Recently, Wang et al. described that simultaneous PET/3 T MRI using 18F-FDG and 68Ga-DOTATATE was useful in the diagnosis and detection of recurrence of pituitary micoadenomas, reducing radiation dose to the patient and improving inter-modality coregistration.[22] Rodriguez-Barcelo et al. showed that software coregistration of 11C-methionine PET images with 3T-MRI could improve the characterization of lesions.[9] Although simultaneous PET/MR has great potential to increase diagnostic performance in specific clinical situations, its use is still limited to specialized centers and its application is currently in the research phase. Software coregistration of PET and MR images is still a valid strategy to enhance diagnostic performance when both modalities are available separately.
The complete quantitative approach to estimate the binding potential of 18F-fallypride requires prolonged dynamic studies that may not be adequate in patients with pituitary and brain tumors. The uptake ratio between the pituitary and the cerebellum has been used to quantify the expression of D2/3 receptors using 18F-fallypride in normal controls and in tumors of the pituitary gland with different radiotracers.[2,3,10,13] This strategy has the limitation of being dependent on the postinjection time at which the analysis is performed, and even though it reveals the expression of D2/3 receptors some degree of nonspecific uptake of the radiotracer cannot be excluded. Mukherjee et al. showed that the ratio between the normal pituitary gland and the cerebellum reaches a plateau at 60 min.[13] In normal controls, the pituitary/cerebellum ratio at this time frame is close to 8, while in the evaluation of pituitary tumors with other radiotracers, tumor/cerebellum rates from 0 to 15 have been described.[2,3,10,13] In our patients, we found tumor/cerebellum ratio from 2.3 to 15.4, showing the high variability of expression of D2/3 receptors that can occur in these tumors.
Although this case series has a limited sample size and cannot be used to assess the correlation of D2/3 expression with clinical outcomes, of note, the patient with low receptor expression and high AA metabolism had to undergo surgery due to a poor response to cabergoline. On the other hand, one of the patients with low receptor expression and low AA metabolism responded well to cabergoline, demonstrating that the biology of the tumor in terms of AA transport and metabolism may have a significant influence in prediction to DAs treatment. Therefore, the potential use of 18F-fallypride in combination with labeled AAs to predict outcomes and response to DAs requires further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chanson P, Maiter D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab. 2019;33:101290. doi: 10.1016/j.beem.2019.101290. [DOI] [PubMed] [Google Scholar]
- 2.Pirker W, Riedl M, Luger A, Czech T, Rössler K, Asenbaum S, et al. Dopamine D2 receptor imaging in pituitary adenomas using iodine-123-epidepride and SPECT. J Nucl Med. 1996;37:1931–7. [PubMed] [Google Scholar]
- 3.De Herder WW, Reijs AE, De Swart J, Kaandorp Y, Lamberts SW, Krenning EP, et al. Comparison of iodine-123 epidepride and iodine-123 IBZM for dopamine D, receptor imaging in clinically non-functioning pituitary macroadenomas and macroprolactinomas. Eur J Nucl Med. 1999;26:46–50. doi: 10.1007/s002590050358. [DOI] [PubMed] [Google Scholar]
- 4.Bergström M, Muhr C, Lundberg PO, Långström B. PET as a tool in the clinical evaluation of pituitary adenomas. J Nucl Med. 1991;32:610–5. [PubMed] [Google Scholar]
- 5.Colao A, Ferone D, Lastoria S, Cerbone G, Di Sarno A, Di Somma C, et al. Hormone levels and tumour size response to quinagolide and cabergoline in patients with prolactin-secreting and clinically non-functioning pituitary adenomas: Predictive value of pituitary scintigraphy with 123I-methoxybenzamide. Clin Endocrinol (Oxf) 2000;52:437–45. doi: 10.1046/j.1365-2265.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias P, Cardona J, Díez JJ. The pituitary in nuclear medicine imaging. Eur J Intern Med. 2019;68:6–12. doi: 10.1016/j.ejim.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Bergström M, Muhr C, Lundberg PO, Bergström K, Lundqvist H, Antoni G, et al. Amino acid distribution and metabolism in pituitary adenomas using positron emission tomography with D-[11C] methionine and L-[11C] methionine. J Comput Assist Tomogr. 1987;11:384–9. doi: 10.1097/00004728-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Tang BN, Levivier M, Heureux M, Wikler D, Massager N, Devriendt D, et al. 11C-methionine PET for the diagnosis and management of recurrent pituitary adenomas. Eur J Nucl Med Mol Imaging. 2006;33:169–78. doi: 10.1007/s00259-005-1882-0. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Barcelo S, Gutierrez-Cardo A, Dominguez-Paez M, Medina-Imbroda J, Romero-Moreno L, Arraez-Sanchez M. Clinical usefulness of coregistered 11C-methionine positron emission tomography/3-T magnetic resonance imaging at the follow-up of acromegaly. World Neurosurg. 2014;82:468–73. doi: 10.1016/j.wneu.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Muhr C, Bergström M, Lundberg PO, Bergström K, Hartvig P, Lundqvist H, et al. Dopamine receptors in pituitary adenomas: PET visualization with 11C-N-methylspiperone. J Comput Assist Tomogr. 1986;10:175–80. doi: 10.1097/00004728-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 11.de Herder WW, Reijs AEM, Feelders RA, van Aken MO, Krenning EP, van der Lely AJ, et al. Diagnostic imaging of dopamine receptors in pituitary adenomas. Eur J Endocrinol. 2007;156((suppl 1)):S53–6. doi: 10.1530/eje.1.02349. [DOI] [PubMed] [Google Scholar]
- 12.Stoessl AJ. Neuroimaging in Parkinson's disease: From pathology to diagnosis. Parkinsonism Relat Disord. 2012;18((suppl 1)):S55–9. doi: 10.1016/S1353-8020(11)70019-0. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: Blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–88. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Cumming P, Son YD, Hang-Keun K, Yo-Han J, Jong-Hoon K, et al. Altered connectivity between striatal and extrastriatal regions in patients with schizophrenia on maintenance antipsychotics: an [18F] fallypride PET and functional MRI study. Synapse. 2018;72:e22064. doi: 10.1002/syn.22064. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Gui S, Cao L, Hua L, Jiwei B, Chuzhong L, et al. Use of micro-positron emission tomography with18F-fallypride to measure the levels of dopamine receptor-D2 and 18F-FDG as molecular imaging tracer in the pituitary glands and prolactinomas of Fischer-344 rats. Onco Targets Ther. 2016;9:2057–68. doi: 10.2147/OTT.S94057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergström M, Muhr C, Lundberg PO, Långström B. PET as a tool in the clinical evaluation of pituitary adenomas. J Nucl Med. 1991;32:610–5. Available from: https://pubmed.ncbi.nlm.nih.gov/2013801/ [PubMed] [Google Scholar]
- 17.Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [18F] fallypride. Synapse. 2010;64:350–62. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laruelle M, Slifstein M, Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imaging Biol. 2003;5:363–75. doi: 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Thorner MO, Schran HF, Evans WS, Rogol AD, Morris JL, MacLeod RM, et al. A broad spectrum of prolactin suppression by bromocriptine in hyperprolactinemic women: A study of serum prolactin and bromocriptine levels after acute and chronic administration of bromocriptine. J Clin Endocrinol Metab. 1980;50:1026–33. doi: 10.1210/jcem-50-6-1026. [DOI] [PubMed] [Google Scholar]
- 20.Wu ZB, Zheng WM, Su ZP, Chen Y, Wu JS, Wang CD, et al. Expression of D2RmRNA isoforms and ERmRNA isoforms in prolactinomas: Correlation with the response to bromocriptine and with tumor biological behavior. J Neurooncol. 2010;99:25–32. doi: 10.1007/s11060-009-0107-y. [DOI] [PubMed] [Google Scholar]
- 21.Seok H, Lee EY, Choe EY, Yang WI, Kim JY, Shin DY, et al. Analysis of 18F-fluorodeoxyglucose positron emission tomography findings in patients with pituitary lesions. Korean J Intern Med. 2013;28:81–8. doi: 10.3904/kjim.2013.28.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hou B, Lu L, Feng M, Zang J, Yao S, et al. PET/MRI in the Diagnosis of Hormone-Producing Pituitary Microadenoma: A Prospective Pilot Study. J Nucl Med. 2018;59:523–8. doi: 10.2967/jnumed.117.191916. [DOI] [PubMed] [Google Scholar]
- 23.Feng Z, He D, Mao Z, Wang Z, Zhu Y, Zhang X, et al. Utility of 11C-methionine and 18F-FDG PET/CT in patients with functioning pituitary adenomas. Clin Nucl Med. 2016;41:e130–4. doi: 10.1097/RLU.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 24.D'Amico A, Stąpór-Fudzińska M, Tarnawski R. CyberKnife radiosurgery planning of a secreting pituitary adenoma performed with 68Ga DOTATATE PET and MRI. Clin Nucl Med. 2014;39:1043–4. doi: 10.1097/RLU.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 25.Parghane RV, Agrawal K, Mittal BR, Shukla J, Bhattacharya A, Mukherjee KK. 68Ga DOTATATE PET/CT in a rare coexistence of pituitary macroadenoma and multiple paragangliomas. Clin Nucl Med. 2014;39:91–3. doi: 10.1097/RLU.0b013e3182a77b78. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Ranade R, Hazarika S. 68Ga DOTATATE PET/CT of Synchronous Meningioma and Prolactinoma. Clin Nucl Med. 2016;41:230–1. doi: 10.1097/RLU.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Xiao J, Xing B, Wang R, Zhu Z, Li F. Comparison of (68) Ga DOTATATE to 18F-FDG uptake is useful in the differentiation of residual or recurrent pituitary adenoma from the remaining pituitary tissue after transsphenoidal adenomectomy. Clin Nucl Med. 2014;39:605–8. doi: 10.1097/RLU.0000000000000457. [DOI] [PubMed] [Google Scholar]