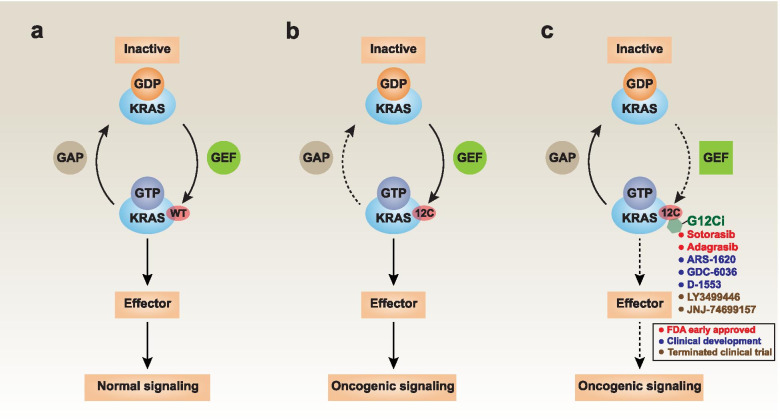
Fig. 2.
Principle of inhibiting oncogenic KRAS activation. a. The wild-type (WT) KRAS protein maintains a balance between the inactive state of guanosine diphosphate (GDP) binding and the active state of guanosine triphosphate (GTP) binding. This process is mediated by GTPase activating protein (GAP) and guanine nucleotide exchange factor (GEF). b. The KRAS oncoprotein (e.g., KRAS-G12C) disrupts GAP-mediated GTP hydrolysis, allowing these mutants to accumulate in a continuous GTP-binding active state, which is responsible for oncogenic activity. c. The covalent inhibitor of KRAS-G12C protein (G12Ci) achieves allosteric inhibition of mutant cysteine 12 (12C) to prevent GEF-catalyzed nucleotide exchange and block subsequent effector pathways