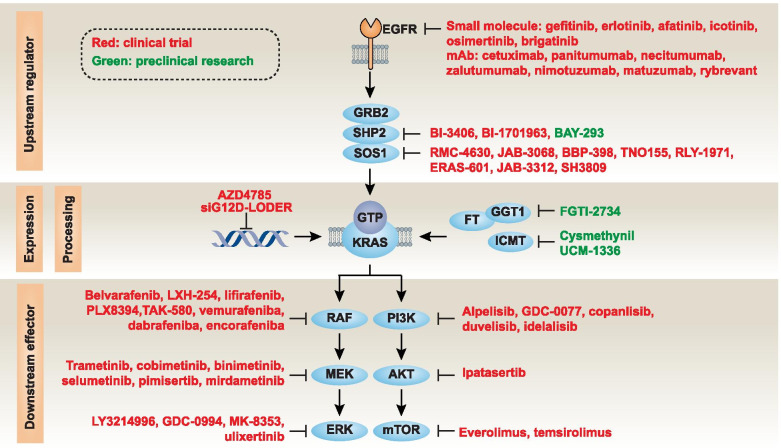
Fig. 3.
Indirect KRAS suppression strategy. The activation of receptor tyrosine kinases, such as members of the epidermal growth factor receptor (EGFR) family, activate KRAS through the growth factor receptor-bound protein 2 (GRB2)-SH2–containing protein tyrosine phosphatase 2 (SHP2)-SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS1) pathway. The mutant KRAS protein accumulates in the guanosine triphosphate (GTP)-bound state, leading to the activation of downstream effector pathways, especially the RAF-MEK-extracellular signal regulated kinase (ERK) and the phosphatidylinositol 3-kinase (PI3K)-AKT-mechanistic target of rapamycin (mTOR) pathways. The localization of KRAS on the cell membrane is the first step in subsequent KRAS activation, which is mediated by enzymes, including but not limited to farnesyltransferase (FT), geranylgeranyltransferase 1 (GGT1), and isoprenylcysteine carboxyl methyltransferase (ICMT). In addition to directly inhibiting KRAS (exemplified by covalent allele-specific inhibitors that bind to KRAS-G12C), multiple approaches can indirectly inhibit the oncogenic pathway of KRAS by targeting upstream regulators, downstream effectors, and KRAS expression and processing. The main drugs or reagents used for indirect KRAS inhibition are shown in red (for clinical trials or approved for use in patients) or green (for preclinical research)