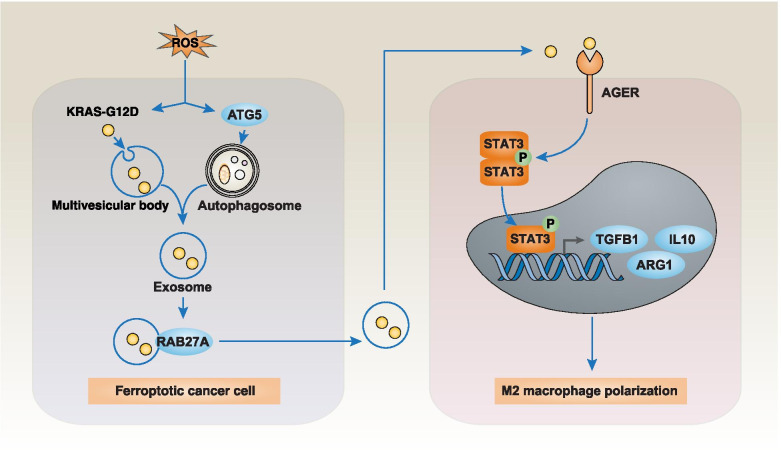
Fig. 4.
The immunosuppressive function of extracellular KRAS-G12D protein in the tumor microenvironment. KRAS-G12D protein can be released during ferroptosis, which is a regulated cell death caused by reactive oxygen species (ROS) and subsequent lipid peroxidation. The release of KRAS-G12D protein is mediated by exosomes, which are cargo extracellular vesicles produced by multivesicular bodies derived from endosomes. The small GTPase RAB27A regulates exocytosis of multivesicular endosomes, which leads to exosome secretion. This process is further enhanced by autophagy-related 5 (ATG5)-dependent autophagosome formation and autophagy-meditated secretion. Once released, the extracellular KRAS-G12D protein from exosomes is taken up by advanced glycosylation end product-specific receptor (AGER) on macrophages, leading to phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3). Nuclear STAT3 acts as a transcription factor to produce cytokines, such as transforming growth factor beta 1 (TGFB1), interleukin 10 (IL10), and arginase 1 (ARG1), for polarization of M2 macrophages, which limits antitumor immunity