Abstract
Objective
Previously published work has indicated that transcripts encoding transglutaminase 2 (TG2) increase markedly in a rat model of abdominal aortic aneurysm. This study determines whether TG2 and the related TG, factor XIII-A (FXIII-A), protect against aortic aneurysm development in mice.
Methods
C57BL/6J wild-type, Tgm2–/– knockout, F13a1–/– knockout, and Tgm2–/–/F13a1–/– double knockout mice were subjected to laparotomy and periaortic application of CaCl2.
Results
Tgm2–/– mice showed slightly greater aortic dilatation at 6 weeks after treatment when compared with wild type. However, vessels from Tgm2–/– mice, but not wild-type mice, continued to dilate up to 6 months after injury and by 24 weeks, a greater number of Tgm2–/– mice had developed aneurysms (16/17 vs 10/19; P = .008). Laparotomy resulted in a high death rate in F13a1–/– knockout mice, more frequently from cardiac complications than from hemorrhage, but among F13a1–/– mice that survived for 6 weeks after CaCl2 treatment, abdominal aortic aneurysm diameter was unaltered relative to wild-type mice. Laparotomy resulted in a higher death rate among Tgm2–/–/F13a1–/– double knockout mice, owing to an increased frequency of delayed bleeding. Surprisingly, Tgm2–/–/F13a1–/– double knockout mice showed a trend toward decreased dilatation of the aorta 6 weeks after injury, and this finding was replicated in Tgm2–/–/F13a1–/– mice subjected to carotid artery injury. Levels of transcripts encoding TG2 were not increased in the aortas of injured wild-type or F13a1–/– knockout mice relative to uninjured mice, although changes in the levels of other transcripts accorded with previous descriptions of the CaCl2 aneurysm model in mice.
Conclusions
Knockout of Tgm2, but not F13a1 exacerbates aortic dilatation, suggesting that TG2 confers protection. However, levels of TG2 messenger RNA are not acutely elevated after injury. FXIII-A plays a role in preventing postoperative damage after laparotomy, confirming previous reports that it prevents distal organ damage after trauma. TG2 promotes wound healing after surgery and, in its absence, the bleeding diathesis associated with FXIII-A deficiency is further exposed.
Keywords: Aneurysm, Transglutaminase 2, Factor XIII-A
Clinical relevance
There is a lack of medical treatments that inhibit the expansion of aneurysms and thus prevent or delay the need for surgical intervention. Transglutaminase 2 (TG2) has been proposed to play a role in the repair of tissues including blood vessels. Our results do not confirm that TG2 expression is acutely increased in response to aneurysm development, but instead suggest that absence of TG2 exacerbates experimental aneurysm development. Pharmacologic induction of TG2 expression may form part of a strategy to exploit the healing of aneurysmal arteries.
Article Highlights.
-
•
Type of Research: Basic science study
-
•
Key Findings:Tgm2–/– mice show a small increase in aortic diameter 6 weeks after CaCl2 injury relative to control mice while vessels from Tgm2–/– mice, but not wild-type mice, continued to dilate up to 24 weeks after injury. Tgm2–/–/F13a1–/– double knockout mice show a trend toward decreased aortic dilatation and this finding was replicated in Tgm2–/–/F13a1–/– mice subject to carotid artery injury.
-
•
Take Home Message: Transglutaminase 2 (TG2) has been proposed to play a role in the repair of blood vessels. Our results do not confirm that TG2 expression is acutely increased after aneurysm development, but instead suggest that the absence of TG2 exacerbates experimental aneurysm development.
Arterial aneurysms exhibit thinning and disruption of the medial layer, resulting from the loss of elastin and vascular smooth muscle cells. Collagen synthesis may increase to stabilize aneurysms, but collagen itself undergoes accelerated degradation, leading to vessel dilatation and eventual rupture.1 Abdominal aortic aneurysms (AAA) are detected in the infrarenal aorta of 1.3% to 3.3% of males aged 65 to 80 years undergoing screening, with risk factors that include hypertension, atherosclerosis, and smoking, and account for 1% to 2% of deaths in this group.1 Both open and endovascular aneurysm repair carry significant risk2 and effective medical treatments that mitigate AAA development are not available currently.3
Targeting the proteases that degrade aortic proteins could potentially moderate aneurysm development. However, several proteases may need to be simultaneously inhibited to halt disease progression,4 and although absence of matrix metalloproteinase (MMP)9 inhibits aneurysm development in animal models,5, 6, 7 administration of the MMP inhibitor, doxycycline, to patients with small aneurysms failed to decrease aneurysm growth.8
Alternatively, it may be possible to promote the expression of enzymes that mediate arterial protection and repair. TGs comprise a family of eight enzymes that introduce Nε (γ-glutamyl) lysine isopeptide cross-links between and within protein chains, conferring mechanical stability and proteolytic resistance.9 Among these, extracellular TG210 cross-links matrix proteins including fibronectin11,12 and possibly isoforms of collagen.11 In addition, intracellular TG2 regulates gene expression and hence decisions of cell fate.13 Although many cells express TG2 basally, expression is induced in response to proinflammatory stimuli14 and when macrophages polarize to a reparative M2 phenotype.15 Accordingly, TG2 is present in injured tissues, such as the vulnerable shoulder regions of human atherosclerotic plaques.16,17
A second TG, blood clotting factor XIII-A (FXIII-A), circulates in plasma as the heterotetramer FXIII-A2B2 and is present within the cytosol of cell types including megakaryocytes, platelets, and macrophages as the homodimer FXIII-A2.18 Plasma FXIII-A2B2 cross-links matrix proteins including fibrin, fibronectin, collagen, and vitronectin19 and contributes to placental maintenance20 and dermal repair.21 Macrophage FXIII-A expression is induced upon differentiation to a reparative M2 phenotype22 and cooperates with plasma FXIII-A2B2 to stabilize myocardial scars23 and with macrophage-derived TG2 to facilitate inward arterial remodelling.24
Munezane et al25 reported that TG2 expression increases when infrarenal aortic aneurysms are induced in rats. We previously observed that lack of TG2 lessens the resistance of carotid arteries to the mechanical strain of ligation.26 Given that weakened vessels are prone to aneurysm development,27 this finding might imply that TG2 would confer protection. We are unaware of evidence that FXIII-A directly influences aneurysm development, although various studies have proposed that FXIII-A overlaps in function with TG2 (eg,28). Therefore, to address the roles of TG2 and FXIII-A in aneurysm development, we have induced aneurysms in the infrarenal aorta ofTgm2–/– knockout, F13a1–/– knockout, and Tgm2–/–/F13a1–/– double knockout mice.
Methods
Animal housing, husbandry, and procedures were conducted in accordance with guidelines and regulations of the University of Leeds and of the United Kingdom Home Office. Mice had ad libitum access to water and to a standard chow diet (softened for 24 to 48 hours after surgery).
Generation of TG-deficient mice
The breeding and genotyping of Tgm2+/– mice29 and of F13a1–/– mice and Tgm2–/–/F13a1–/– mice each back-crossed onto a more than 97.5% C57BL/6J background26 have been described previously. Equal numbers of male and female mice at 8 to 10 weeks of age were used for each procedure.
Harvesting and biochemical analysis of arteries
Mice were anaesthetized with isoflurane and then subjected to perfusion exsanguination via the left ventricle with 5 mL phosphate-buffered saline (Sigma Aldrich, Dorset, UK). The descending aorta to the iliac bifurcation was snap frozen in liquid N2, stored at –80°C, and either (i) processed to determine messenger RNA (mRNA) levels26 or (ii) dehydrated to constant weight under vacuum at 80°C, lysed for 48 hours at 4°C in 750 μL of Na2HPO4 (50 mmol.L−1), NaCl (50 mmol.L−1), 1% Triton X-100, 0.1% SDS, pH 7.4, and subjected to centrifugation (14,000×g for 10 minutes at 4°C). The supernatant fraction was assayed to determine protein (bicinchoninic acid assay; Sigma-Aldrich) and lactate dehydrogenase activity (CytoTox 96 lactate dehydrogenase assay; Promega, Madison, Wisc). DNA was assayed after forming a fluorescent complex for 2 hours with Sybr green I gel stain (1:20,000 dilution of stock solution [ThermoFisher Scientific, Waltham, Mass], in 50 mmol.L−1 Na2HPO4, 2 mol.L−1 NaCl, pH 7.4, λex = 493 nm, λem = 530 nm). Pellets were used to measure oxalic acid-soluble elastin or pepsin-soluble collagen (Fastin or Sircol assays, respectively; Biocolor, Carrickfergus, County Antrim, UK).
Wire myography
Abdominal aortas (n = 4) were dissected in cold Hanks buffered salt solution30 and two rings from each (1 mm in length) were mounted in a myograph (610 mol/L; Danish Myograph Technology, Hinnerup, Denmark), equilibrated for 30 minutes, and then placed under normalized tension in Krebs–Henseleit buffer gassed with 5% CO2/95% O2 at 37°C.31 The contractile responses to KCl (60 mmol.L−1) and to phenylephrine (1 μmol.L−1) were verified, after which the response to increasing concentrations of phenylephrine was determined. The median effective concentration values were estimated using Origin software (OriginLab Corporation, Northampton, Mass). Finally, endothelial integrity was confirmed by showing that carbachol (1 μmol.L−1) induced relaxation of vessels that had contracted in response to phenylephrine (1 μmol.L−1).
Aneurysm induction
Mice were anaesthetized and then either the abdominal aorta was exposed or the right common carotid artery was exposed and isolated from surrounding tissue using a silicone strip (Eddingtons Ltd, Hungerford, UK). Subsequently CaCl2 (0.5 mol.L−1) or NaCl (0.15 mol.L−1) was applied to the artery (2 × 7 minutes), and the exposed area was rinsed with NaCl (0.15 mol.L−1). The mice received an intraperitoneal injection of Buprenorphine 0.1 to 1.0 mg/kg (Vetergesic, Reckitt Benckiser, Slough, UK) before recovery. Arteries were imaged in situ before CaCl2 treatment and at termination (6 weeks or 24 weeks) using the OPMI-PICO video micrometer (Carl Zeiss AG, Jena, Germany). Vessel measurements were independently determined by two investigators using Image-Pro software (MediaCybernetics, Rockville, Md).
Histology
Arteries for histologic examination were perfusion-fixed in 4% paraformaldehyde in 50 mmol.L−1 Na2HPO4, 150 mmol.L−1 NaCl, pH 7.4. Serial transverse sections were cut at 5-μm intervals from the left renal artery to iliac bifurcation and stained with Miller's elastic Van Gieson, hematoxylin and eosin, picrosirius red, or alizarin red S and were imaged using an Olympus BX61WI inverted microscope with an XC10-IR camera under the control of CellSens software (Olympus, Tokyo, Japan). Fibrillar collagen density adjacent to damaged and undamaged regions from Van Gieson stained aortic sections was determined by excitation at 800 nm with a Chameleon laser and collecting the second harmonic signal (400 nm) through a 10× objective lens and an EF SP 485 IR++ filter onto a Zeiss LSM NDD R2 detector using a Zeiss 710 multiphoton microscope and analyzing 10 × 10 pixel square regions using ImageJ.
All image analysis was carried out in a blinded manner.
Quantification of messenger RNA
Mouse aortas (n = 8-10) were disrupted in TRIzol (ThermoFisher Scientific) using a TissueLyser II (Qiagen, Hilden, Germany), The nuclei acid fraction was precipitated from the aqueous layer and mRNA processed for reverse transcriptase polymerase chain reaction, as previously described,26 using the primer pairs shown in the Supplementary Table (online only). Transcript levels were normalized to those encoding ribosomal protein subunit-32, using the 2–ΔCt method.32
Immunohistochemistry
Immunofluorescent detection of CD163 and FXIII-A antigens in sections of mouse aorta was carried out as previously described.33
Statistical analysis
Unless stated, all are is presented as mean ± standard deviation. All values were analyzed using Prism 7 (GraphPad software, San Diego, Calif). Comparisons between each group were carried out using the unpaired Student t test (with Welch's correction) or one-way analysis of variance (with Bonferroni correction) for multiple groups. A Kruskal-Wallis test with Dunn's multiple comparison test was used for any data that were not normally distributed; normality was assessed using Shapiro-Wilk testing. Contingency data (proportion of mice developing an aneurysm; defined as increase in diameter of >50%) was analyzed using Fisher's exact test of proportions. Significance was accepted where the P was less than .05.
Results
Baseline properties of mouse aortas are similar between genotypes
The mRNAs encoding TG2, FXIII-A. and TG3 were the most abundant TG transcripts in the wild-type aorta. Other TG mRNAs were present at very low levels or were undetectable (TG6). Knockout of the F13a1 gene, the Tgm2 gene, or both did not result in induction of other Tgm genes to a level expected to compensate for either gene knockout. Immunofluorescence confirmed that FXIII-A was present in CD163 positive macrophages of aorta (Fig 1), as previously described in skin34 and heart.33
Fig 1.
Transglutaminase (TG) expression in aortas from C57Bl/6J wild-type mice and transglutaminase knockout mice. Transcripts encoding TG2 and factor XIII-A (FXIII-A) were detected in C67Bl/6J wild-type (WT) mice but were absent from the relevant knockout mice. Transcripts encoding TG3 were detectable in WT mice but did not increase in knockout mice. Transcripts encoding TG1, TG4, TG5, and TG7 were present in WT mouse aortas at very low concentrations relative to TG2 expression (main graph) and did not increase in Tgm2–/– or F13a1–/– knockout mice (lower inset). FXIII-A protein was present in CD163 positive cells (macrophages) in WT mice, but was not detected in CD163 macrophages in F13a1–/– knockout mice (upper inset). mRNA, Messenger RNA.
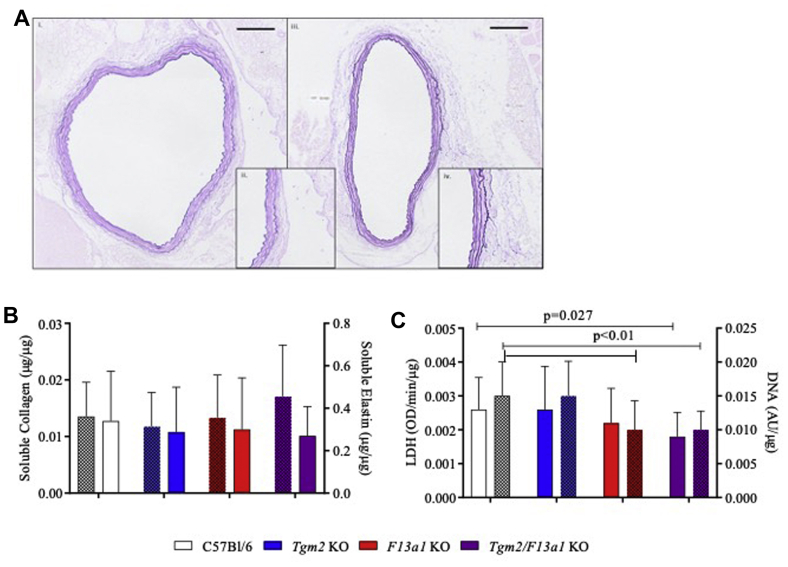
The aortic structure was similar between genotypes, illustrated by representative images of wild-type and Tgm2–/–/F13a1–/– aortas (Fig 2, A). The mean lamellar number (n = 4) did not differ between genotypes (C57Bl/6J, 4.76 ± 0.54; Tgm2–/–, 4.38 ± 0.49; F13a1–/–, 4.60 ± 0.49; Tgm2–/–/F13a1–/–, 4.64 ± 0.54). Measurements of elastin were consistent with published values35,36 and did not vary between genotypes. Pepsin-soluble collagen was measured by the Biocolor assay, which preferentially detects newly synthesized collagen, and showed no statistically significant difference between groups (Fig 2, B). Elastin and collagen could not be assayed in the same sample, precluding estimation of errors in the ratio. However the ratio (average (soluble collagen/protein)/(average [soluble elastin/protein]) seemed to be higher in Tgm2–/–/F13a1–/– mice (0.0633) than in other genotypes (C57Bl/6J, 0.0398; Tgm2–/–, 0.0458; F13a1–/–, 0.0455 [n = 10-12]). DNA content and lactate dehydrogenase activity, measured as indices of cellularity, were decreased in F13a1–/– and Tgm2–/–/F13a1–/– aortas (Fig 2, C).
Fig 2.
Mice lacking factor XIII-A (FXIII-A) have decreased aortic cellularity but similar elastic morphology. A, Representative Miller's elastic Van Gieson stained sections (5 μm) from a basal (unoperated) aorta from (i and ii) a C57Bl/6J wild-type mouse and (iii and iv) a Tgm2–/–/F13a1–/– mouse. Insets to show lamellar structure; scale bar represents 100 μm. B, Concentrations of oxalate-soluble elastin (plain bars) and of pepsin-soluble collagen (patterned bars) expressed per microgram of SDS-soluble protein in C57Bl/6J and transglutaminase (TG) knockout mouse aortas. Neither protein varied in concentration between mice of different genotypes (n = 8-12). C, Activity of lactate dehydrogenase (LDH) (plain bars) and concentration of DNA (patterned bars) in the aorta (n = 25-28). Both LDH and DNA content were decreased in Tgm2–/–/F13a1–/– mice and DNA content was decreased in F13a1–/– knockout mice.
The basal tension exerted by excised aortas from Tgm2–/–/F13a1–/– mice tended to be lower than that exerted by wild-type, Tgm2–/– or F13a1–/– mice (P = .072). The median effective concentration for the phenylephrine response and the maximal increase in aortic tension were similar between genotypes (Fig 3). In summary, while the properties of aortas from F13a1–/– and Tgm2–/– single knockout mice appeared similar to C57Bl/6J wild-type mice, there were detectable differences in the basal state of Tgm2–/–/F13a1–/– double knockout aortas that could affect their response to aneurysm development.
Fig 3.
Ex vivo function of aortas from wild-type and transglutaminase (TG) knockout mice. A, Increase in force exerted by excised aortas in response to increasing concentrations of phenylephrine (PE). B, Calculated concentrations (median effective concentration [EC50]) at which contraction generated half maximal force. C, Area under the curves (AUC) from experiments in (A). There was a tendency for contraction to be weaker in Tgm2–/–/FXIII-A–/– mice at all concentrations of PE, resulting in a lower AUC. ANOVA, Analysis of variance; KO, Knockout.
Laparotomy is followed by failure to thrive and high mortality in mice lacking FXIII-A
Despite recovering from surgery, a high proportion of F13a1–/– mice failed to regain weight and died, often within 1-5 days (Fig 4, A, B). Necropsy frequently showed blackened, thrombus-filled atria (Fig 4, C) and/or multiple discrete areas of focal necrosis in the bowel, spleen, and liver. Postoperative mortality was further increased in Tgm2–/–/F13a1–/– mice over F13a1–/– mice (Table) and the increase was largely attributable to delayed bleeding. A comparable death rate among NaCl-treated (sham-operated) Tgm2–/–/F13a1–/– mice indicated that deaths were not a consequence of aneurysm development.
Fig 4.
Mice lacking factor XIII-A (FXIII-A) show increased mortality after laparotomy. A,F13a1–/– knockout (KO) and Tgm2–/–/F13a1–/– double knockout mice showed lower survival after laparotomy than C57Bl/6J wild-type mice. End represents scheduled termination date at 6 weeks. B, The average change in body weight of mice after laparotomy. Mice that would survive (S) regained weight to within 5% of baseline by 7 days. Mice that would not survive for 6 weeks after the operation (NS) showed little or no weight gain. C, Cardiac abnormalities present in a representative F13a1–/– mouse after laparotomy, including a dusky myocardium. Upon opening, thrombus was detected within the right atrial cavity (not shown). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Table.
Mortality and aneurysm development rate in mice following periaortic application of CaCl2 (0.5 mol.L−1 or NaCl (0.15 mol.L−1)
| Genotype | Overall operative mortality (%) | Causes of death (% of deaths within group) | Aneurysm prevalence at 6 -weeks after injury | Aneurysm prevalence at 24 weeks after injury | |
|---|---|---|---|---|---|
| C57Bl/6J | NaCl | 9.4a | - | - | |
| CaCl2 | 17/28 (60.7%) | 10/19 (52.6%) | |||
| Tgm2–/– | NaCl | 3.4 | - | - | |
| CaCl2 | 20/27 (74.1%) | 17/17 (94.1%) | |||
| F13a1–/– | NaCl | 37.3 | Bleeding 11% Cardiac +/- thrombotic 37% |
- | - |
| CaCl2 | 16/26 (61.5%) | - | |||
| Tgm2–/–/F13a1–/– | NaCl | 62.0 | Bleeding 27% Cardiac +/- thrombotic 27% |
- | - |
| CaCl2 | 7/19 (36.8%) | - |
Values are number/total number (%) unless otherwise indicated.
Aneurysm prevalence (absolute numbers and percentage) of mice developing an aneurysm (defined as aortic dilatation >50%) in each genotype group. Vessel dilatation was defined at the time of harvest at either 6 or 24 weeks after CaCl2 injury.
The mortality of wild-type mice undergoing CaCl2 injury or NaCl sham procedure includes the first mice operated upon during familiarization with the method. The mortality rate among Tgm2–/– mice (3.4%) more accurately reflects the incidental death rate among wild-type mice post-familiarization. Deaths post-familiarization were very high in F13a1–/– mice and Tgm2–/–/F13a1–/– mice. Other causes of death include fighting post-operation, bowel ischemia and wound complications. A large renal mass was detected in one Tgm2–/–/F13a1–/– mouse. In some cases, the cause of death was unknown.
Knockout of Tgm2 exacerbates aneurysm progression, but FXIII-A does not exhibit functional redundancy with TG2
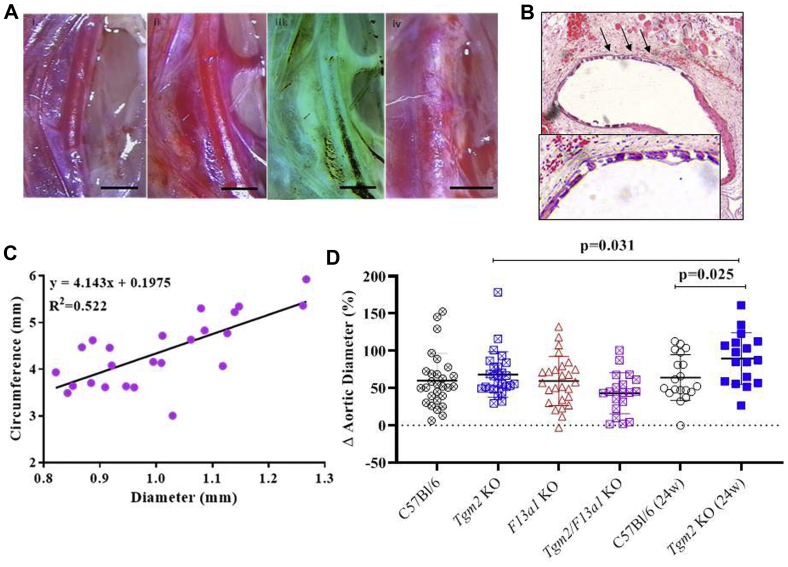
Exposure to CaCl2 caused surface inflammation and an increase in external aortic diameter in all genotypes (Fig 5, A). Transverse sections showed elastic fiber breakage (Fig 5, B) and were used to measure internal vessel circumference. Regression analysis indicated that the ratio Δ(internal circumference)/Δ(external diameter) was 4.1 (Fig 5, C), which confirmed that luminal dilatation contributed to the increase in external diameter (expected value = π). Although a regression analysis cannot exclude that wall thickening also contributed to the increase in external diameter, this finding was not apparent by visual inspection of the sections, whereas wall thickening in the absence of dilatation would have generated a line of zero gradient. Tgm2–/– mice showed slightly greater aortic dilatation 6 weeks after treatment (67.5 ± 30.2%) than wild-type mice (60.0 ± 36.3%; P = .097) (Fig 5, D). Aneurysms further progressed in Tgm2–/– mice in the period up to 24 weeks (89.5 ± 34.5% dilatation [P = .025] relative to Tgm2–/– mice at 6 weeks), but showed minimal progression in wild-type mice (64.1 ± 30.2% dilatation at 24 weeks [P = .57] relative to wild-type at 6 weeks). Defining aneurysms as a 50% increase in aortic diameter,37 16 of the 17 Tgm2–/– mice developed aneurysms at 24 weeks as opposed to 10 of the 19 wild-type mice (P = .008).
Fig 5.
A, Representative images of aortas from wild-type mice captured: (i) before treatment; (ii) 6 weeks after the application of 0.15 mol.L−1 NaCl, native image; (iii) contrast inversion of image (ii); and (iv) 6 weeks after the application of 0.5 mol.L−1 CaCl2. Scale bars represent 1 mm. Treatment with CaCl2, but not NaCl, induced surface inflammation and focal dilatation. Contrast inversion afforded better resolution of the vessel margins and facilitated the measurement of the external diameter. B, Transverse section (stained with hematoxylin and eosin and imaged at 10× original magnification) of a C57Bl/6J wild-type aorta obtained 6 weeks after exposure to 0.5 mol.L−1 CaCl2. The ventral face (marked with arrows and shown in the inset) displays elastin breakage and fiber loss. C, The external diameter of CaCl2-treated arteries from wild-type mice is linearly related to the vessel circumference, confirming luminal dilatation. D, The percentage change in aortic diameter from baseline (mean ±95% confidence intervals) is shown at 6 weeks (crossed symbol) and 24 weeks (24 weeks, filled symbol) after treatment with 0.5 mol.L−1 CaCl2. There was no statistically significant difference between genotypes at 6 weeks of age (analysis of variance; P = .097), although there was a tendency for dilatation to be less in Tgm2–/–/F13a1–/– double knockout mice. At 24 weeks, the mean aortic diameter had further increased in the Tgm2–/– mice (6 weeks vs 24 weeks; P = .025), causing the average diameter to be greater than that in the C57Bl/6J wild-type mice (P = .031). KO, Knockout.
In view of the possibility that FXIII-A might act redundantly with TG2 to influence aneurysm development, aortic dilatation was also measured 6 weeks after CaCl2 treatment in F13a1–/– mice and Tgm2–/–/F13a1-/- mice. Dilatation was essentially equal in F13a1–/– mice (59.4 ± 32.9%) and wild-type mice, but tended to be less in Tgm2–/–/F13a1–/– mice (43.1 ± 27.5%; P = .097). Although the cause of death after laparotomy in Tgm2–/–/F13a1–/– mice seemed to be unrelated to aneurysm development, it seemed possible that Tgm2–/–/F13a1–/– mice that would otherwise have developed a large AAA were more likely to die prematurely than those developing a small aneurysm. To address this phenomenon, we also induced aneurysms in the carotid artery and all mice survived 6 weeks after CaCl2 treatment. Similar to the abdominal aorta, dilatation of the carotid artery was less in Tgm2–/–/F13a1–/– mice (20.7 ± 15.9%) than wild-type mice (43.1 ± 34.8%; P = .028) (Fig 6), suggesting that the decrease in aortic dilatation in Tgm2–/–/F13a1–/– mice was not biased by mortality. Although the basis for this unexpected decrease in dilatation is unclear, the results exclude the possibility that FXIII-A acts redundantly with TG2 to inhibit aneurysm protection.
Fig 6.
Tgm2–/–/F13a1–/– mice show decreased dilatation of the carotid artery after application of CaCl2. A, Representative images of the carotid injury model of a C57Bl/6J wild-type mouse before (left) and after (right) treatment with 0.5 mol.L−1 CaCl2. Scale bars represent 500 μm. B, The percent change from baseline in the external diameter of carotid artery 6 weeks after treatment with 0.5 mol.L−1 CaCl2 is greater in C57Bl/6J wild-type than in Tgm2–/–/F13a1–/– double knockout (KO) mice (P = .028). C, Representative transverse sections (5 μm) of a C57Bl/6J wild-type carotid artery 6 weeks after application of 0.5 mol.L−1 CaCl2, and stained with (i) hematoxylin and eosin stain and (ii) Miller's elastic Van Gieson stain. Elastic flattening and breakage were apparent in wild-type and Tgm2–/–/F13a1–/– (not shown) carotid arteries following injury. Scale bar represents 100 μm.
In view of the high mortality associated with laparotomy in mice lacking FXIII-A, and the lack of evidence that FXIII-A protects against aneurysm development, aneurysm development at 24 weeks was not assessed in either F13a1–/– or Tgm2–/–/F13a1–/– mice.
Collagen density is decreased adjacent to areas of elastic breakage and is decreased in Tgm2–/–mice relative to wild-type mice
The fractional areas within aneurysms of wavy elastin (normal), straightened elastin (damaged), or broken elastin 6 weeks after CaCl2 treatment did not differ significantly between genotypes (Fig 7, A). However, elastin breakage tended to be decreased in Tgm2–/–/F13a1–/– mice in accord with their reduced dilatation. In each genotype, the density of fibrillar collagen was decreased adjacent to regions of elastic fiber flattening and was further decreased adjacent to regions of elastic fiber breakage (Fig 7, B, C). Fibrillar collagen density at regions of similar damage did not differ between genotypes after 6 weeks, but was decreased adjacent to intact and flattened regions in 24-week aneurysms in Tgm2–/– mice (relative to wild-type mice), consistent with the increased aneurysm size seen at this time point.
Fig 7.
Collagen density is decreased adjacent to areas of medial damage. A, There is no significant difference between genotypes in the percentage distribution of regions of apparently healthy media, straightened elastic lamina, or broken elastic lamina in aortas examined 6 weeks after CaCl2 treatment. B, Representative Miller's elastic Van Gieson stained images of the media (above) and second harmonic images of fibrillar collagen in the adjacent adventitia (below) in areas in which the lamina appeared: (i) healthy, (ii) straightened or (iii) broken. C, The density of fibrillar collagen, measured in arbitrary units (AU) by second harmonic signal at 400 nm decreased adjacent to areas of laminar damage in mice of all genotypes, but there is no difference between genotypes 6 weeks after CaCl2 treatment (plain bars). However, when measured 24 weeks after CaCl2 treatment, the collagen density is significantly reduced in Tgm2 knockout (KO) mice relative to wild-type mice, adjacent to areas of healthy and straightened elastin (patterned bars).
Despite evidence that either FXIII-A or TG2 activity is necessary for ectopic calcification,38 fragmented elastin underwent calcification39 in all genotypes including Tgm–/–/F13a1–/– mice. Calcification persisted for at least 24 weeks in wild-type (Fig 8, A) and Tgm2–/– (not shown) mice.
Fig 8.
Medial degeneration is associated with calcification in treated mouse aortas. A, Sections of mouse aorta obtained 6 weeks or 24 weeks after treatment with CaCl2 and stained with hematoxylin and eosin (H&E), Miller's elastic Van Gieson (MVG) stain, and alizarin red S for deposition of calcium. Intense staining for calcium is apparent at the injured ventral face of the aorta in all genotypes 6 weeks after CaCl2 treatment. Representative images from wild-type and Tgm2–/–/F13a1–/– double knockout (KO) mice are shown. Calcium deposition was also apparent 24 weeks after CaCl2 deposition, when in some mice, medial degeneration and calcification were apparent around the whole circumference of the vessel. B, Aorta from two representative C57Bl/6J wild-type mice examined 24 hours after CaCl2 treatment. Alizarin red S staining showed occasional small yellow orange Ca2+ containing crystals in the lamellae, but not widespread Ca2+ deposition, verifying that CaCl2 treatment did not immediately flood the aorta with Ca2+ ions, but initiated tissue damage.
Gene expression studies revealed marked alterations in aneurysmal arteries from mice lacking FXIII-A
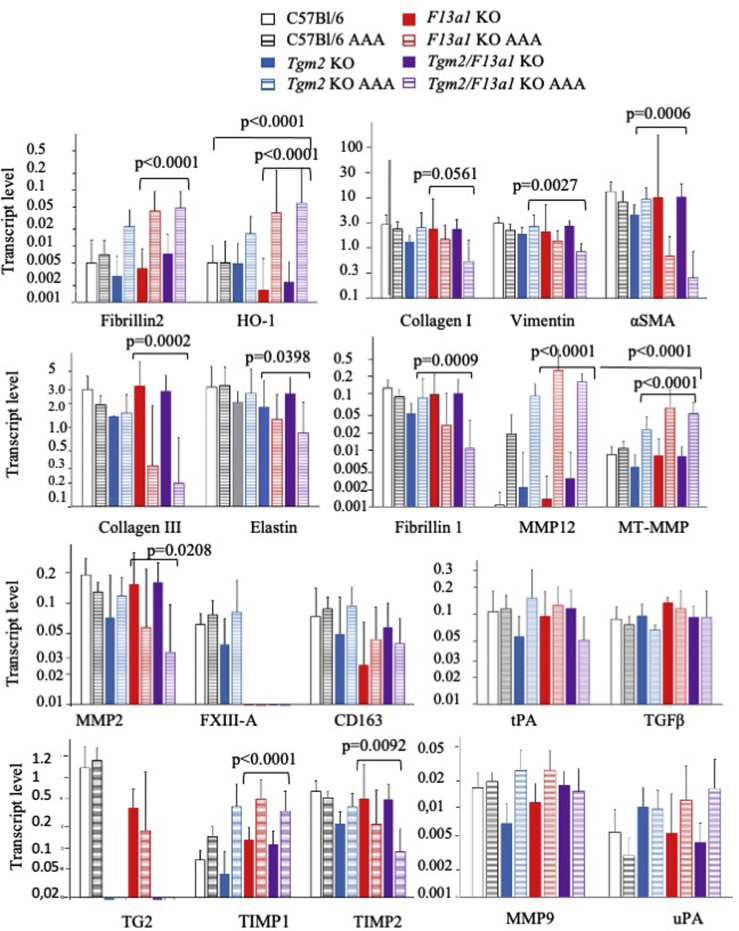
Because visual inspection could not easily determine the boundary between injured and uninjured tissue, RNA was isolated from the aorta extending from the aortic arch to the iliac bifurcation to ensure comparability between samples. Serial sectioning suggested that at least 25% of the excised tissue was injured (not shown). Apart from the targeted genes in knockout mice, transcript levels did not show consistent differences between genotypes. Normalized levels of transcripts including MMP12, MT1-MMP (MMP14), and heme oxygenase increased after aneurysm development, particularly in the gene knockout mice, consistent with the invasion of inflammatory macrophages (Fig 9). In contrast, transcripts encoding FXIII-A and CD163 (present in resident macrophages) did not show statistically significant changes in expression. Further, TG2 mRNA did not increase after aneurysm induction in either wild-type or F13a1–/– mice (Fig 9).
Fig 9.
Transcript levels in aortas in control and aneurysmal aortas. Transcript levels in aortas collected 6 weeks after CaCl2 treatment were determined by reverse transcriptase polymerase chain reaction and normalized to the levels of ribosomal protein large subunit (Rpl)32. Mean values are shown with 95% confidence intervals. Data are presented on a logarithmic scale for clarity. Statistically significant increases upon aneurysm induction were observed in messenger RNAs (mRNAs) encoding heme oxygenase 1, matrix metalloproteinase 12 (MMP12) and membrane-type MMP (MT-MMP), comparing aneurysmal and nonoperated aortas mice from all genotypes. For other transcripts including CD163, tissue type plasminogen activator (tPA), transforming growth factor (TGF)-β, no significant changes in levels were observed between aneurysmal and non-operated aortas. Surprisingly marked decreases in mRNAs encoding α-smooth muscle actin (α-SMA) and collagen III were seen in both Tgm2–/–/F13a1–/– mice and F13a1–/– mice and significant decreases in mRNAs encoding elastin and MMP2 were also apparent in these genotypes. Probability values shown here compare aneurysmal and nonaneurysmal aortas from all mice deficient in factor XIII-A (FXIII-A). HO-1, Heme oxygenase 1; TG, Transglutaminase.
Transcripts including vimentin, smooth muscle α-actin and collagen III (possibly also collagen I; P = .056) showed decreases in aneurysmal arteries from both F13a1 knockout and Tgm2/F13a1 double knockout mice relative to uninjured aortas from mice of the same genotypes. MMP2 mRNA was also decreased in injured relative to uninjured aortas in these mice, whereas MMP9 showed no significant difference in the levels between the genotypes. Fibrillin I mRNA levels decreased, whereas fibrillin 2 mRNA increased in FXIII-A-deficient mice, suggesting reversion to a more fetal pattern of gene expression40; there were also reciprocal changes between TIMP1 (expression increased in gene knockout mice) and TIMP2 (expression decreased) (Fig 9).
The mRNAs for TG1 and TG7 increased in each of the three mouse gene knockout lines (P < .01 in each case), but the low initial concentrations of these mRNAs, relative to the mRNAs encoding TG2 and FXIII-A (Fig 1), mean that it is unlikely that they had increased sufficiently to compensate for either knockout. Although TG3 was detected at appreciable levels in uninjured aorta, no statistically significant changes in TG3 mRNA were observed upon aneurysm induction (P > .7).
Discussion
The risk that AAA poses to human health has prompted the development of several small animal models, including induction of AAA by periarterial application of CaCl2.37,41 Munezane et al25 reported that aneurysms of the rat infrarenal aorta induced by combined CaCl2 and luminal elastase treatment showed a marked rise in TG2 expression and suggested that this might constitute a protective response.
Here we have tested whether TG2 influences CaCl2-induced AAA development by comparing wild-type and Tgm2–/– mice. Tgm2–/– mice showed a marginal increase in dilatation relative to wild-type mice 6 weeks after CaCl2 treatment, but dilatation then continued from 6 to 24 weeks in the Tgm2–/– but not wild-type mice, suggesting a role for TG2 in arterial protection or repair. Because FXIII-A might act redundantly with TG2 to mediate protection or repair, we also induced aneurysms in Tgm2–/–/F13a1–/– and F13a1–/– mice. Dilatation was unaltered in F13a1 mice, whereas, in contrast with expectations, dilatation was decreased in Tgm2–/–/F13a1–/– mice.
We cannot account for the decreased dilatation of aortas from Tgm2–/–/F13a1–/– mice, although the basal tension and the elastin/collagen ratio of their aortas appeared different from other genotypes. Tgm2–/–/F13a1–/– mice also develop red blood cell extravasation and myocardial fibrosis,26 whereas altered fibronectin metabolism was noted in a separate cohort of Tgm2–/–/F13a1–/– mice.28 It is unclear whether common factors underlie the fibrosis, alterations in fibronectin metabolism, and the arterial changes seen here. Regardless of this finding, our results show that FXIII-A does not act redundantly with TG2 to protect against aneurysm development in the CaCl2 model.
Human aneurysms frequently exhibit a decreased elastin/collagen ratio, owing to preferential degradation of elastin coupled, in some instances, with increased collagen deposition.27,42 We observed lower periaortic collagen density adjacent to the injured face of mouse aneurysms, both at 6 and 24 weeks after injury, suggesting that compensatory collagen deposition had not occurred. Periaortic collagen density was also lower at 24 weeks in Tgm2–/– mice than in wild-type mice, corresponding with the increased dilatation in these animals. A decrease in TG2-mediated cross-linking and consequent increase in susceptibility to proteolysis could explain the decreased density of collagen in the Tgm2–/– mice, although verifying this finding by quantifying isopeptide cross-links remains difficult.43,44 Alternatively, the absence of intracellular TG2 may have altered cellular function13 and hence the deposition of collagen.
Although we observed calcification of mouse AAA, rat AAA did not calcify in the study of Munezane et al25 and this difference in aneurysm morphology was accompanied by differences in gene expression profiles. In particular, we did not observe increased levels of mRNAs encoding TG2, MMP2, or MMP9,25 although similar to Longo et al45 we observed an increase in MMP12 mRNA. Our results indicate that an increase in TG2 mRNA levels is not an invariable response to vessel dilatation, and similarly using a microarray approach, Biros et al46 found that TG2 mRNA did not increase within human AAA, but that it did increase in aortic obstructive disease. We have stained AAA sections from four patients with a polyclonal antibody to TG2 and have observed intense areas of striated staining in isolated areas of inflammation, but also large areas devoid of staining (not shown). Therefore, localized expression of TG2 could be important in the response to disease, even if an average increase in mRNA expression across the whole lesion is not evident
A proportion of mice that lacked FXIII-A died within a few days of laparotomy. Necropsy frequently revealed cardiac pathology (eg, atrial thrombosis) in C57Bl/6J F13a1–/– mice, sometimes associated with distal ischemia, and with clots that may have embolized from the heart. In addition to this pathology, Tgm2–/–/F13a1–/– mice showed an increased frequency of hemorrhage relative to F13a1–/– mice, that may have resulted from delayed bleeding caused by surgical injury or postoperative stress. It seems that compromised tissue protection and repair in the absence of TG2 exposes the bleeding diathesis associated with FXIII-A deficiency, similar to the situation where TG2 deficiency exacerbates extravasation and myocardial fibrosis associated with FXIII-A deficiency.26 The cause of the nonhemorrhagic deaths is uncertain, but FXIII-A also maintains endothelial barrier function26,47 and can protect against systemic organ injury.48,49 Impaired barrier function may have rendered F13a1 knockout mice susceptible to cardiac damage after laparotomy. We also observed large decreases in the mRNAs encoding certain structural proteins after aneurysm induction, particularly in mice lacking FXIII-A and note that Kothapalli et al50 observed decreases of similar magnitude in collagen and elastin expression in cells explanted from CaCl2-induced rat aortic aneurysms, although presumably these cells expressed FXIII-A. Further work is needed to determine whether common pathways underlie the unexpected changes in gene expression in aneurysmal aortas from F13a1–/– knockout mice and the changes in endothelial barrier function, and hence mouse survival.
Of the three models commonly used to induce AAA in mice, the relevance of the angiotensin II-hyperlipidemia protocol has been questioned.51 The infusion of elastase into the aortic lumen causes rapid dilatation, but would risk hemorrhage in mice lacking FXIII-A. Further, ligation necessary for elastase infusion is expected to preferentially damage Tgm2–/– vessels,26 complicating the analysis of aneurysm development. We therefore chose the CaCl2 protocol to minimize manipulation of the aorta. A limitation of the CaCl2 model is that murine aneurysms do not accumulate luminal thrombus, possibly because of rapid fibrinolysis.52,53 Second, CaCl2-induced aneurysms rarely rupture and cannot model protection against this outcome. Third, mice do not express proelafin/trappin-2, a neutrophil elastase inhibitor, which is cross-linked by TG(s) to the extracellular matrix54 and which may confer protection in human arterial lesions.17
A point of difference between the C57BL/6J mice used here and the mixed strain mice used previously is that ligation caused carotid artery rupture in 50% of mixed strain apoE–/–/Tgm2–/–/F13a1–/– triple knockout mice and elastic breakage without rupture in apoE–/–/Tgm2–/– mice,26 whereas ligation did not cause carotid rupture in either C57BL/6J Tgm2–/–/F13a1–/– or C57BL/6J apoE–/–/Tgm2–/–/F13a1–/– mice (n = 10). Nevertheless, ligation induced the deposition of additional elastic lamellae in C57BL/6J mice lacking TG2 (not shown), presumably to reinforce the intrinsically weaker vessel against mechanical stress, and showing that the elastase protocol might prove problematic in these mice. We suspect that rupture depended on the additional stress caused by neointimal deposition, which was occlusive in the mixed strain mice, but, as reported,55,56 was minimal or absent in C57BL/6J mice.
Regardless of these caveats, we conclude that the basal level of TG2 expression in aorta appears sufficient to limit mouse AAA development. We did not detect induction of TG2 mRNA expression upon injury, but do not exclude the possibility that localized increases in TG2 expression may have occurred but were not apparent in total tissue measurements. Experiments were not done that would confirm that the replacement or enhancement of TG2 would be protective; however, because TG2 expression is one of a limited number of enzymes implicated in tissue repair (and its expression can be induced pharmacologically10), further studies are merited to determine whether TG2 may prove a future viable target for the management of human AAA.
Author contributions
Conception and design: KG, CJ, DS, RP
Analysis and interpretation: KG, LN, SI, CJ, RP
Data collection: KG, KS, CB, LC, NY
Writing the article: KG, RP
Critical revision of the article: KS, CB, LN, LC, NY, SI, CJ, DS
Final approval of the article: KG, KS, CB, LN, LC, NY, SI, CJ, DS, RP
Statistical analysis: KG, KS, CB
Obtained funding: KG, DS, RP
Overall responsibility: RP
Appendix.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only).
Supplementary Table (online only).
Primer sequences for reverse transcriptase polymerase chain reaction
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Elastin | AAAGCCTGGGAAAGTTCCTG | TACACCTGGAAGACCAACAC |
| Col I | ATGGATTCCCGTTCGAGTACG | TCAGCTGGATAGCGACATCG |
| Col III | CACCCTTCTTCATCCCACTCTTA | ACCAAGGTGGCTGCATCC |
| Vimentin | CGGAAAGTGGAATCCTTGCA | CACATCGATCTGGACATGGCTGT |
| aSMA | ACTGGGACGACATGGAAAAG | GTTCAGTGGTGCCTCTGTCA |
| MMP2 | CTGATAACCTGGATGCCGTCGT | TGCTTCCAAACTTCACGCTCTT |
| MMP9 | GTCTCGGGAAGGCTCTGCTGTT | CTCTGGGGATCCACCTTCTGAG |
| FXIII-A | TGCTGGTGTCTTTAACACATTTTTAA | TGGGCCGAGAATGAATTGGT |
| TG1 | TTCGCTACCCGTACCGTCA | CTTCATCCAGCAGTCGTT |
| TG2 | ATTGGCAGRGRGGACATTC | TCGTGGGCGGAGTTGTA |
| TG3 | AAGAAGCTGACCATGAGTGCTTT | TGCGCCCTTCGATTCATAG |
| TG4 | CCCATCTATTTGACCATAACTTGAA | GCGAGAAACACCCTTGATT |
| TG5 | AGGCAGGATTCTGGAGAATATG | GGGCCACAGCCACAGCAGTAGAG |
| TG6 | TCCGAGTCAAT GTGAGCG | GTCTTCTGTCAGGTCTCCTTTGTA |
| TG7 | ATGTGCACGGTAATGAGATGCT | TGTGTGCAGAATGGAAATTGG |
| CD163 | ATGGGTGGACACAGAATGGTT | CAGGAGCGTTAGTGACAGCAG |
| tPA | CAACAGCGGCCTGGTACAA | CCCCATTGAAGCATCTTGGTT |
| uPA | GAAACCCTACAATGCCCACAGA | GACAAACTGCCTTAGGCCAATC |
| PAI-1 | ACGGTGATGCGATATAATGTAAACG | CATTCCTGAGAACACAGCATTG |
| Fibrillin 1 | CCTGTGCTATGATGGGTTCA | AGGTCCCACTAAGGCAGATG |
| Fibrillin 2 | CCACTCCTATTGCTGCCCAG | TTGGGGCGGGAACAGAATC |
| MT-MMP | CCCAAGGCAGCAACTTCA | CAATGGCAGCTGAGAGTGAC |
| HO-1 | AACAAGCAGAACCCAGTCTATG | TGAGCAGGAAGGCGGTCTTA |
| MMP12 | GCTAGAAGCAACTGGGCAAC | ACCGCTTCATCCATCTTGAC |
| TIMP1 | GTGGGAAATGCCGCAGCAGAT | GGGCATATCCACAGAGGCTTT |
| TIMP2 | CCAGAAGAAGAGCCTGAACCA | GTCCATCCAGAGGCACTCATC |
| TGFB | CACCGGAGAGCCCTGGATA | TGTACAGCTGCCGCACACA |
| B-Actin | CGTGAAAAGATGACCCAGATCA | TCGTACGACCAGAGGCATACAG |
| RPL32 | AAAATTAAGCGAAACTGGCGG | TGTTGCTCCCATAACCGATG |
Except for mouse factor XIII-A, primers to mouse transglutaminases were as described by Johnson et al.1 Mouse factor XIII-A primers spanning the boundary of coding exons 7 and 8 (mRNA exons 8 and 9), were designed using Primer Express. Other primers were obtained from primer bank (http://pga.mgh.harvard.edu/primerbank/).
References
- 1.Nordon I.M., Hinchliffe R.J., Loftus I.M., Thompson M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner I., Hirsch A.T., Abola M.T., Cacoub P.P., Poldermans D., Steg P.G. Cardiovascular risk profile and outcome of patients with abdominal aortic aneurysm in out-patients with atherothrombosis: data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. J Vasc Surg. 2008;48:808–814. doi: 10.1016/j.jvs.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Golledge J., Norman P.E., Murphy M.P., Dalman R.L. Challenges and opportunities in limiting abdominal aortic aneurysm growth. J Vasc Surg. 2017;65:225–233. doi: 10.1016/j.jvs.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Hussien H., Soekhoe R.G., Weber E., von der Thusen J.H., Kleemann R., Mulder A. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyo R., Lee J.K., Shipley J.M., Curci J.A., Mao D., Ziporin S.J. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo G.M., Xiong W., Greiner T.C., Zhao Y., Fiotti N., Baxter B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howatt D.A., Dajee M., Xie X., Moorleghen J., Rateri D.L., Balakrishnan A. Relaxin and matrix metalloproteinase-9 in angiotensin II-induced abdominal aortic aneurysms. Circ J. 2017;81:888–890. doi: 10.1253/circj.CJ-17-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxter B.T., Matsumura J., Curci J.A., McBride R., Larson L., Blackwelder W. Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: a randomized clinical trial. JAMA. 2020;323:2029–2038. doi: 10.1001/jama.2020.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iismaa S.E., Mearns B.M., Lorand L., Graham R.M. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- 10.Jin X., Stamnaes J., Klock C., DiRaimondo T.R., Sollid L.M., Khosla C. Activation of extracellular transglutaminase 2 by thioredoxin. J Biol Chem. 2011;286:37866–37873. doi: 10.1074/jbc.M111.287490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z., Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012;42:939–949. doi: 10.1007/s00726-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang R., Khosla C. Substrates, inhibitors, and probes of mammalian transglutaminase 2. Anal Biochem. 2020;591:113560. doi: 10.1016/j.ab.2019.113560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsukawa H., Furutani Y., Hitomi K., Kojima S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016;7:e2244. doi: 10.1038/cddis.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayardo M., Punzi F., Bondar C., Chopita N., Chirdo F. Transglutaminase 2 expression is enhanced synergistically by interferon-gamma and tumour necrosis factor-alpha in human small intestine. Clin Exp Immunol. 2012;168:95–104. doi: 10.1111/j.1365-2249.2011.04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez F.O., Helming L., Milde R., Varin A., Melgert B.N., Draijer C. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121:e57–e69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 16.Auld G.C., Ritchie H., Robbie L.A., Booth N.A. Thrombin upregulates tissue transglutaminase in endothelial cells: a potential role for tissue transglutaminase in stability of atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2001;21:1689–1694. doi: 10.1161/hq1001.097063. [DOI] [PubMed] [Google Scholar]
- 17.Sumi Y., Inoue N., Azumi H., Seno T., Okuda M., Hirata K. Expression of tissue transglutaminase and elafin in human coronary artery: implication for plaque instability. Atherosclerosis. 2002;160:31–39. doi: 10.1016/s0021-9150(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 18.Muszbek L., Bereczky Z., Bagoly Z., Komaromi I., Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931–972. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 19.Mosher D.F., Schad P.E., Vann J.M. Cross-linking of collagen and fibronectin by factor XIIIa. Localization of participating glutaminyl residues to a tryptic fragment of fibronectin. J Biol Chem. 1980;255:1181–1188. [PubMed] [Google Scholar]
- 20.Koseki-Kuno S., Yamakawa M., Dickneite G., Ichinose A. Factor XIII A subunit-deficient mice developed severe uterine bleeding events and subsequent spontaneous miscarriages. Blood. 2003;102:4410–4412. doi: 10.1182/blood-2003-05-1467. [DOI] [PubMed] [Google Scholar]
- 21.Inbal A., Lubetsky A., Krapp T., Castel D., Shaish A., Dickneitte G. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94:432–437. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 22.Torocsik D., Bardos H., Nagy L., Adany R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol Life Sci. 2005;62:2132–2139. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahrendorf M., Hu K., Frantz S., Jaffer F.A., Tung C.H., Hiller K.H. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation. 2006;113:1196–1202. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker E.N., Pistea A., Spaan J.A., Rolf T., de Vries C.J., van Rooijen N. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res. 2006;99:86–92. doi: 10.1161/01.RES.0000229657.83816.a7. [DOI] [PubMed] [Google Scholar]
- 25.Munezane T., Hasegawa T., Suritala, Tanaka A., Okada K., Okita Y. Activation of transglutaminase type 2 for aortic wall protection in a rat abdominal aortic aneurysm formation. J Vasc Surg. 2010;52:967–974. doi: 10.1016/j.jvs.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 26.Griffin K.J., Newell L.M., Simpson K.R., Beckers C.M.L., Drinkhill M.J., Standeven K.F. Transglutaminase 2 limits the extravasation and the resultant myocardial fibrosis associated with factor XIII-A deficiency. Atherosclerosis. 2020;294:1–9. doi: 10.1016/j.atherosclerosis.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deguchi J.O., Huang H., Libby P., Aikawa E., Whittaker P., Sylvan J. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest. 2009;89:315–326. doi: 10.1038/labinvest.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mousa A., Cui C., Song A., Myneni V.D., Sun H., Li J.J. Transglutaminases factor XIII-A and TG2 regulate resorption, adipogenesis and plasma fibronectin homeostasis in bone and bone marrow. Cell Death Differ. 2017;24:844–854. doi: 10.1038/cdd.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanda N., Iismaa S.E., Owens W.A., Husain A., Mackay F., Graham R.M. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 30.Hanks J.H., Wallace R.E. Relation of oxygen and temperature in the preservation of tissues by refrigeration. Proc Soc Exp Biol Med. 1949;71:196–200. doi: 10.3181/00379727-71-17131. [DOI] [PubMed] [Google Scholar]
- 31.Quan A., Leung S.W., Lao T.T., Man R.Y. 5-hydroxytryptamine and thromboxane A2 as physiologic mediators of human umbilical artery closure. J Soc Gynecol Investig. 2003;10:490–495. doi: 10.1016/s1071-5576(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 32.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Beckers C.M.L., Simpson K.R., Griffin K.J., Brown J.M., Cheah L.T., Smith K.A. Cre/lox studies identify resident macrophages as the major source of circulating coagulation factor XIII-A. Arterioscler Thromb Vasc Biol. 2017;37:1494–1502. doi: 10.1161/ATVBAHA.117.309271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaba L.C., Fuentes-Duculan J., Steinman R.M., Krueger J.G., Lowes M.A. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer G.M., Llaurado J.G. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19:394–399. doi: 10.1161/01.res.19.2.394. [DOI] [PubMed] [Google Scholar]
- 36.Hosoda Y., Kawano K., Yamasawa F., Ishii T., Shibata T., Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Krishna S., Golledge J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis. 2013;226:29–39. doi: 10.1016/j.atherosclerosis.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Vanbavel E., Bakker E.N. A vascular bone collector: arterial calcification requires tissue-type transglutaminase. Circ Res. 2008;102:507–509. doi: 10.1161/CIRCRESAHA.108.173013. [DOI] [PubMed] [Google Scholar]
- 39.Basalyga D.M., Simionescu D.T., Xiong W., Baxter B.T., Starcher B.C., Vyavahare N.R. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Votteler M., Berrio D.A., Horke A., Sabatier L., Reinhardt D.P., Nsair A. Elastogenesis at the onset of human cardiac valve development. Development. 2013;140:2345–2353. doi: 10.1242/dev.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka A., Hasegawa T., Chen Z., Okita Y., Okada K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. J Vasc Surg. 2009;50:1423–1432. doi: 10.1016/j.jvs.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 42.Baxter B.T., Davis V.A., Minion D.J., Wang Y.P., Lynch T.G., McManus B.M. Abdominal aortic aneurysms are associated with altered matrix proteins of the nonaneurysmal aortic segments. J Vasc Surg. 1994;19:797–802. doi: 10.1016/s0741-5214(94)70004-4. discussion: 3. [DOI] [PubMed] [Google Scholar]
- 43.Johnson G.V., LeShoure R., Jr. Immunoblot analysis reveals that isopeptide antibodies do not specifically recognize the epsilon-(gamma-glutamyl)lysine bonds formed by transglutaminase activity. J Neurosci Methods. 2004;134:151–158. doi: 10.1016/j.jneumeth.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Arike L., Hansson G.C., Recktenwald C.V. Identifying transglutaminase reaction products via mass spectrometry as exemplified by the MUC2 mucin - pitfalls and traps. Anal Biochem. 2020;597:113668. doi: 10.1016/j.ab.2020.113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longo G.M., Buda S.J., Fiotta N., Xiong W., Griener T., Shapiro S. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Biros E., Gabel G., Moran C.S., Schreurs C., Lindeman J.H., Walker P.J. Differential gene expression in human abdominal aortic aneurysm and aortic occlusive disease. Oncotarget. 2015;6:12984–12996. doi: 10.18632/oncotarget.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noll T., Wozniak G., McCarson K., Hajimohammad A., Metzner H.J., Inserte J. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999;189:1373–1382. doi: 10.1084/jem.189.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaets S.B., Xu D.Z., Lu Q., Feketova E., Berezina T.L., Gruda M. Recombinant factor XIII diminishes multiple organ dysfunction in rats caused by gut ischemia-reperfusion injury. Shock. 2009;31:621–626. doi: 10.1097/SHK.0b013e31818bbe21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaets S.B., Xu D.Z., Lu Q., Feketova E., Berezina T.L., Malinina I.V. Recombinant factor XIII mitigates hemorrhagic shock-induced organ dysfunction. J Surg Res. 2011;166:e135–e142. doi: 10.1016/j.jss.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kothapalli C.R., Taylor P.M., Smolenski R.T., Yacoub M.H., Ramamurthi A. Transforming growth factor beta 1 and hyaluronan oligomers synergistically enhance elastin matrix regeneration by vascular smooth muscle cells. Tissue Eng Part A. 2009;15:501–511. doi: 10.1089/ten.tea.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trachet B., Aslanidou L., Piersigilli A., Fraga-Silva R.A., Sordet-Dessimoz J., Villanueva-Perez P. Angiotensin II infusion into ApoE-/- mice: a model for aortic dissection rather than abdominal aortic aneurysm? Cardiovasc Res. 2017;113:1230–1242. doi: 10.1093/cvr/cvx128. [DOI] [PubMed] [Google Scholar]
- 52.Tsakiris D.A., Scudder L., Hodivala-Dilke K., Hynes R.O., Coller B.S. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- 53.Bond A.R., Jackson C.L. The fat-fed apolipoprotein E knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. J Biomed Biotechnol. 2011;2011:379069. doi: 10.1155/2011/379069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guyot N., Zani M.L., Maurel M.C., Dallet-Choisy S., Moreau T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry. 2005;44:15610–15618. doi: 10.1021/bi051418i. [DOI] [PubMed] [Google Scholar]
- 55.Harmon K.J., Couper L.L., Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostergren C., Shim J., Larsen J.V., Nielsen L.B., Bentzon J.F. Genetic analysis of ligation-induced neointima formation in an F2 intercross of C57BL/6 and FVB/N inbred mouse strains. PLoS One. 2015;10:e0121899. doi: 10.1371/journal.pone.0121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary Reference
- 1.Johnson K.A., Polewski M., Terkeltaub R.A. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]