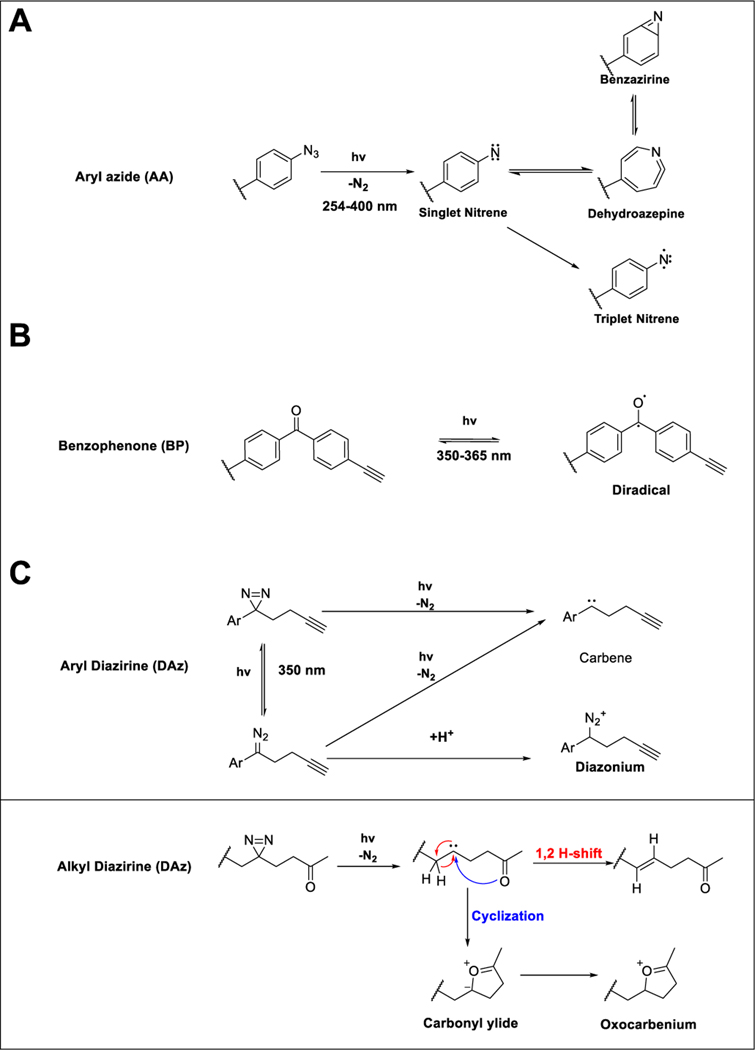
Figure 2.
(A) Photolysis of aryl azide to a singlet nitrene, which can either relax to a triplet nitrene or isomerize to a dehydroazepine or benzazirine. (B) Photolysis of benzophenone to a triplet diradical. (C) Photolysis of diazirine to a carbene or diazo species, which can subsequently lose nitrogen to form the reactive carbene or gain a proton to form a diazonium species. The carbene from alkyl diazirines can further self-quench via a 1,2 H-shift. Alternatively, if the handle bears a carbonyl the carbene can undergo cyclization forming a carbonyl ylide or oxocarbenium species.