Figure 1. Signaling pathways implicated in the perturbation of glutamate homeostasis in the VTA induced by AMP.
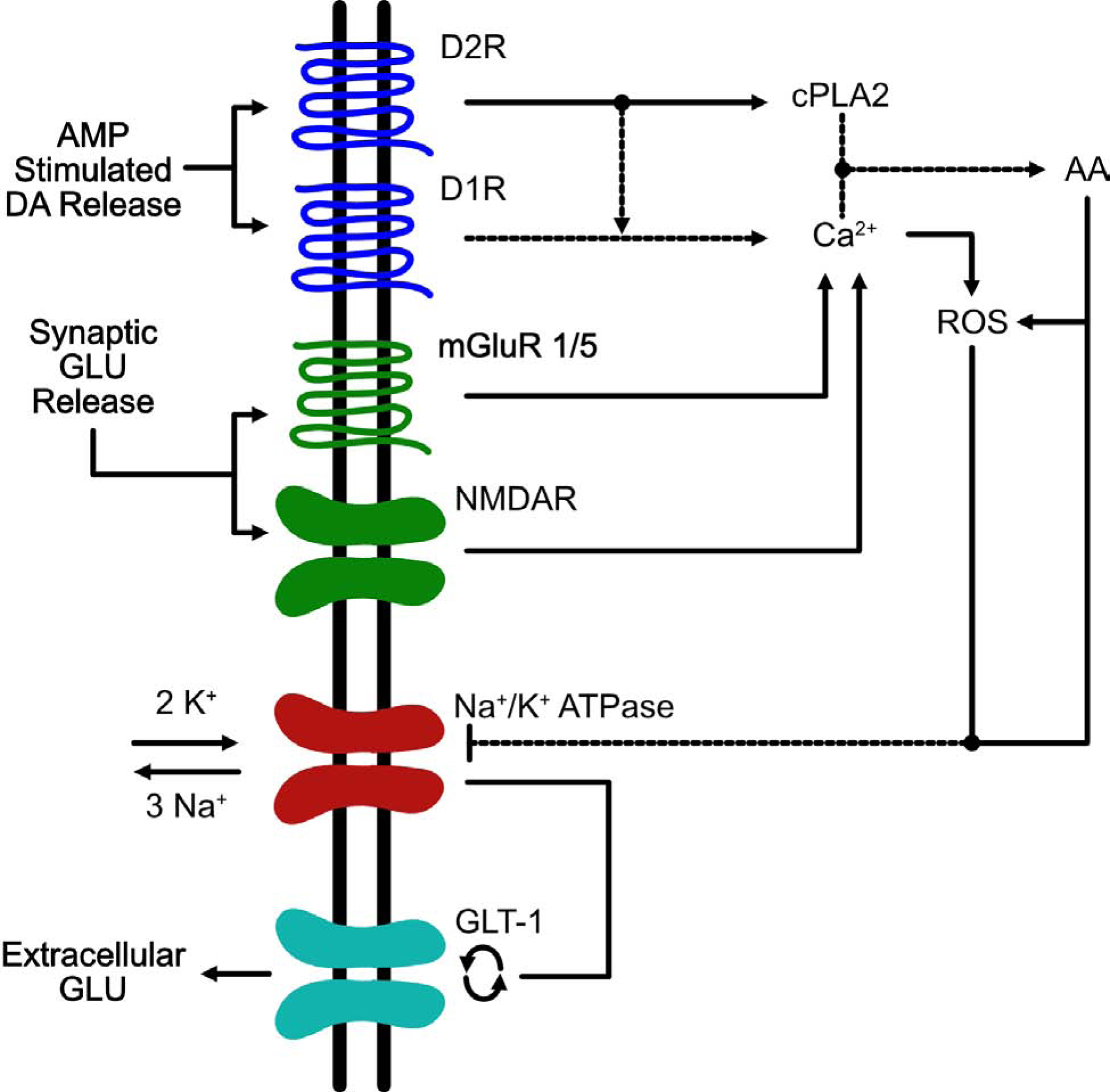
This is an example of a VTA astrocyte in which AMP/METH activates a signaling cascade that ultimately results in the reversal of EAATs. Administration of amphetamines results in the reversal of the dopamine transporter DAT and a decrease in vesicular uptake via VMAT which ultimately results in an accumulation of extracellular levels of DA within multiple brain regions including the VTA (Sulzer and Rayport 1990, Giorgetti, Hotsenpiller et al. 2001). DA released by DAT reversal stimulates D1- and D2-like receptors on VTA astrocytes (Liu, Wang et al. 2009, Zhang, Zhou et al. 2009). Activation of D2 receptors results in activation of the effector enzyme, cytoplasmic phospholipase 2 (cPLA2) (Vial and Piomelli 1995, Bhattacharjee, Chang et al. 2006). The combined stimulation of D1/D2R increases the release of intracellular Ca2+ which along with cPLA2 helps to free arachidonic acid (AA) from phospholipid membranes (Lee, So et al. 2004). AA release results in increased levels of reactive oxygen species (ROS) (Chan and Fishman 1980, Chan, Chen et al. 1988, Sakuma, Kitamura et al. 2012). Both AA and ROS inhibit the Na+/K+ pump (Na+/K+ -ATPase) leading to an increase in cytosolic potassium and a decrease in cytoplasmic sodium (Hexum and Fried 1979, Chan, Kerlan et al. 1983, Volterra, Trotti et al. 1994). The inhibition of Na+/K+ -ATPase results in two events: 1 – depolarization of the cell membrane and 2 – disruption of the Na+/K+ gradients; both of these events can result in the reversal of EAATs (Nicholls and Attwell 1990, Volterra, Trotti et al. 1994, Anderson, Huguenard et al. 2010). The increase in extracellular GLU following AMP administration is most likely due to reversal of GLT-1, as the increases in GLU are completely blocked by the GLT-1 blocker, DHK (Wolf, Xue et al. 2000). Another source of increased extracellular GLU within the VTA arises from GLU input from the PFC. DA projections from the VTA to the PFC play a critical role in modulating PFC GLU output back to the VTA (Sesack and Pickel 1992). By increasing the activity of VTA neurons, AMP/METH could activate this VTA-PFC-VTA circuit resulting in increased extracellular GLU levels within the VTA and in fact it has been shown that acute AMP results in increased activation of this circuit following AMP administration in rats (Colussi-Mas, Geisler et al. 2007). Furthermore, increased levels of extracellular GLU in VTA can activate mGluR1/5 and NMDARs located on the astrocyte resulting in a further increase in intracellular Ca2+ and AA (Biber, Laurie et al. 1999, Daniels and Brown 2001, Lalo, Pankratov et al. 2006, Lee, Ting et al. 2010) which continues the cycle of increases in ROS, decreases in Na+/K+ -ATPase function, and increases in extracellular levels of GLU via reversal of GLT-1.
