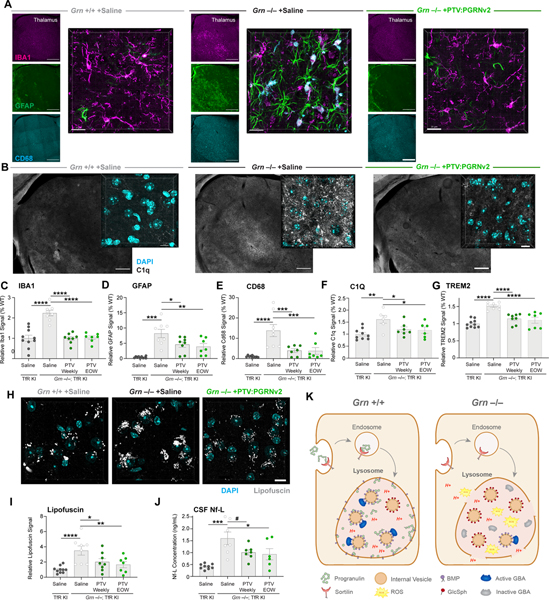
Figure 7: PTV:PGRNv2 rescues gliosis, lipofuscin and neurodegeneration.
(A-F) Representative images and quantification of markers of microgliosis (IBA1), astrogliosis (GFAP) and reactive microglia (CD68, and C1Q), in thalamus of 9mo. Grn−/−; TfRmu/hu mice after weekly or EOW dosing with PTV:PGRNv2 for 8 weeks, as shown in Fig. 6E (n=6–10/group; one way ANOVA, Dunnett’s multiple comparison). (A) Low (left) and high (right) magnification images of thalamic gliosis markers after weekly PTV:PGRNv2 treatment. Left scale bar: 500 μm, right scale bar: 20μm. (B) Low (left) and high (right) magnification images of C1Q expression in thalamus and rescue with weekly PTV:PGRNv2 treatment. Left scale bar: 500 μm, right scale bar: 10μm. Increased total gliosis as measured by IBA1 (C), astrogliosis marker GFAP (D), and reactive microgliosis markers CD68 (E) and C1Q (F) are rescued by PTV:PGRNv2. (G) Reduction of total brain TREM2 levels measured by MSD in Grn−/−; TfRmu/hu by PTV:PGRNv2. (H-I) Representative images (H) and quantification (I) of thalamic lipofuscin in Grn−/−; TfRmu/hu and rescue by PTV:PGRNv2 (n=7–10/group; one way ANOVA, Dunnett’s multiple comparison). (J) Reduced CSF Nf-L levels by PTV:PGRNv2 in Grn−/−; TfRmu/hu mice (n=6–9/group; one way ANOVA, Dunnett’s multiple comparison). (K) Model for PGRN deficiency driven lysosomal dysfunction. Under normal conditions, PGRN is delivered to lysosomes via interaction with sortilin (or indirectly via prosaposin, not shown). There, it is proteolytically cleaved into granulin peptides. PGRN and/or GRN peptides stabilize BMP via both direct physical interaction and prevention of ROS mediated oxidation. This preserves the stimulatory function of BMP toward GCase activity, possibly via saposin C peptides (not shown). In the context of Grn LoF, lysosomal PGRN and GRN peptide deficits result in destabilization of BMP, and vulnerability of lysosomal limiting membrane to oxidative damage, ultimately leading to membrane permeabilization and proton leak. Additionally, depletion of BMP disrupts the interaction of GCase enzyme with the surface of internal vesicles. Impairing lipase activity and driving accumulation of the Gcase substrate GlcSph. #p<0.1, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data shown as mean±SEM. See also Fig. S7.