Figure 2.
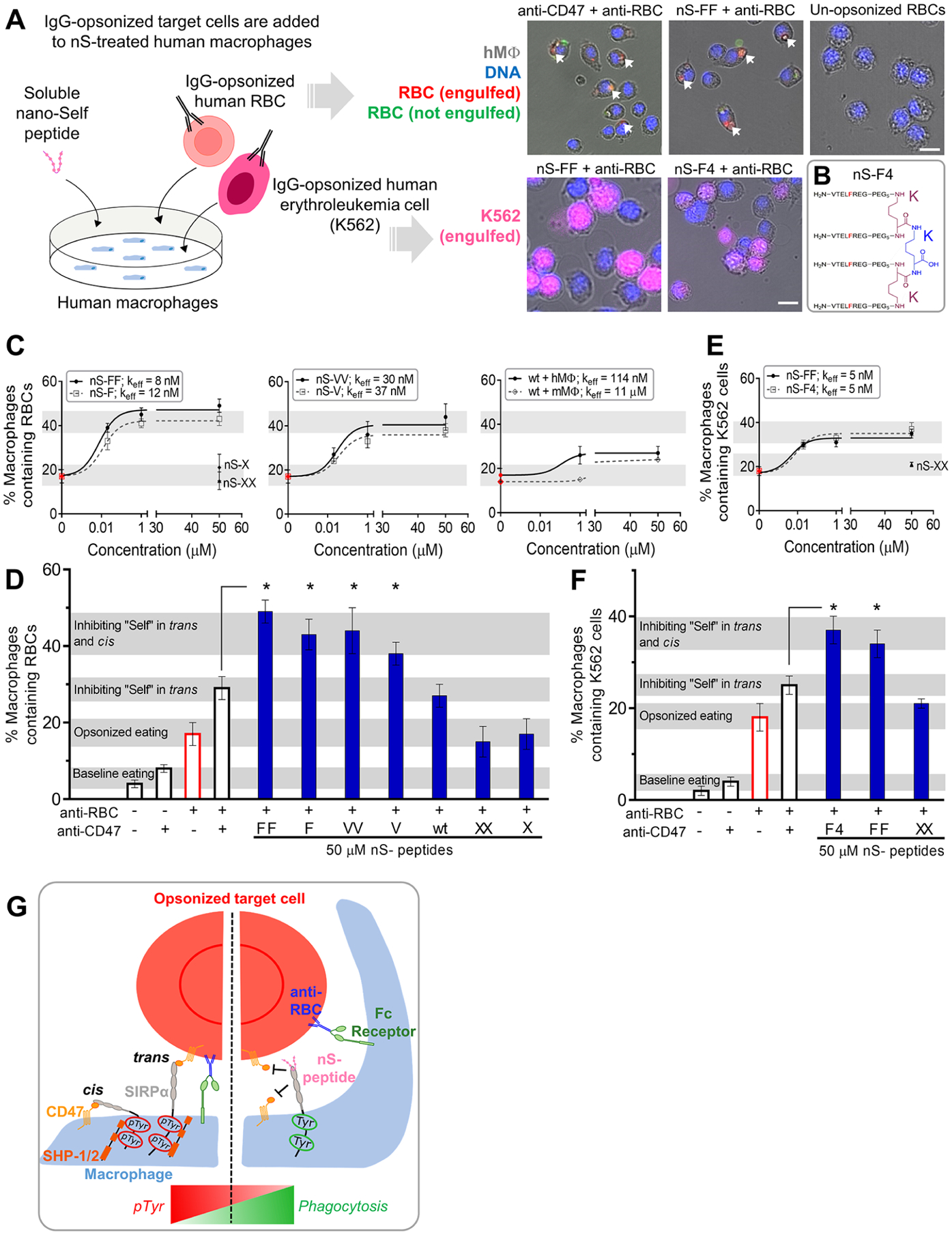
Multivalent nano-Self peptides enhance human macrophage phagocytosis of IgG opsonized targets. (A) Schematic of the phagocytosis assay. RBC and K562 erythroleukemia cell opsonization by anti-human IgG is followed by addition of the cell suspensions to cultured macrophages togther with the soluble nS peptides. Phagocytosis is measured by counting macrophages with internalized, fluorescent target cells (scale bar: 25 μm). (B) Sketch of the tetravalent nS-F4 which consists of a core lysine (blue K) coupled via two lysines (red K’s) with four amines coupled to nS-F’s. (C) Incubating various concentrations of nS peptides with macrophages results in varying levels of macrophages that internalize at least one IgG-opsonized RBC. Relative to nS-wt, suitable substitutions of the key Thr enhanced phagocytosis as did multivalency. Scrambled nS-X or nS-XX peptides do not affect phagocytosis with IgG alone (red points). Phagocytosis by mouse macrophages is also affected by nS-wt, albeit not as much as with human macrophages. At least 200 macrophages were analyzed per condition (hMφ: human macrophages; mMφ: mouse macrophages; n = 3 ± SEM). (D) Saturating macrophages with nS-FF, nS-F, and nS-VV enhances phagocytosis by an additional ~10–20% relative to anti-CD47 treatment of opsonized RBCs. nS-wt is least effective but gives the same result as anti-CD47 and exceeds opsonization alone (n = 3 ± SEM; * denotes p < 0.05 relative to CD47-blocked and opsonized RBCs). (E) Phagocytosis of IgG-opsonized K562 erythroleukemia cells is enhanced by nS-FF and nS-F4. (F) Saturating macrophages with nS-FF and nS-F4 increases phagocytosis of opsonized K562 cancer cells relative to opsonized and CD47 blocked cells, but nS-XX has no effect on IgG-driven engulfment. (G) Schematic of how nS peptides engage and enhance phagocytosis. Left panel: CD47-expressing cells signal “self” through trans engagement with SIRPα on the macrophage, increasing pTyr signals, and overriding prophagocytic signaling from the opsonizing IgG. Right panel: nS peptides in solution engage with SIRPα, inhibiting trans binding of CD47 on opsonized cells and also inhibiting cis binding on the macrophages, leading to increased phagocytosis.
