Figure 3.
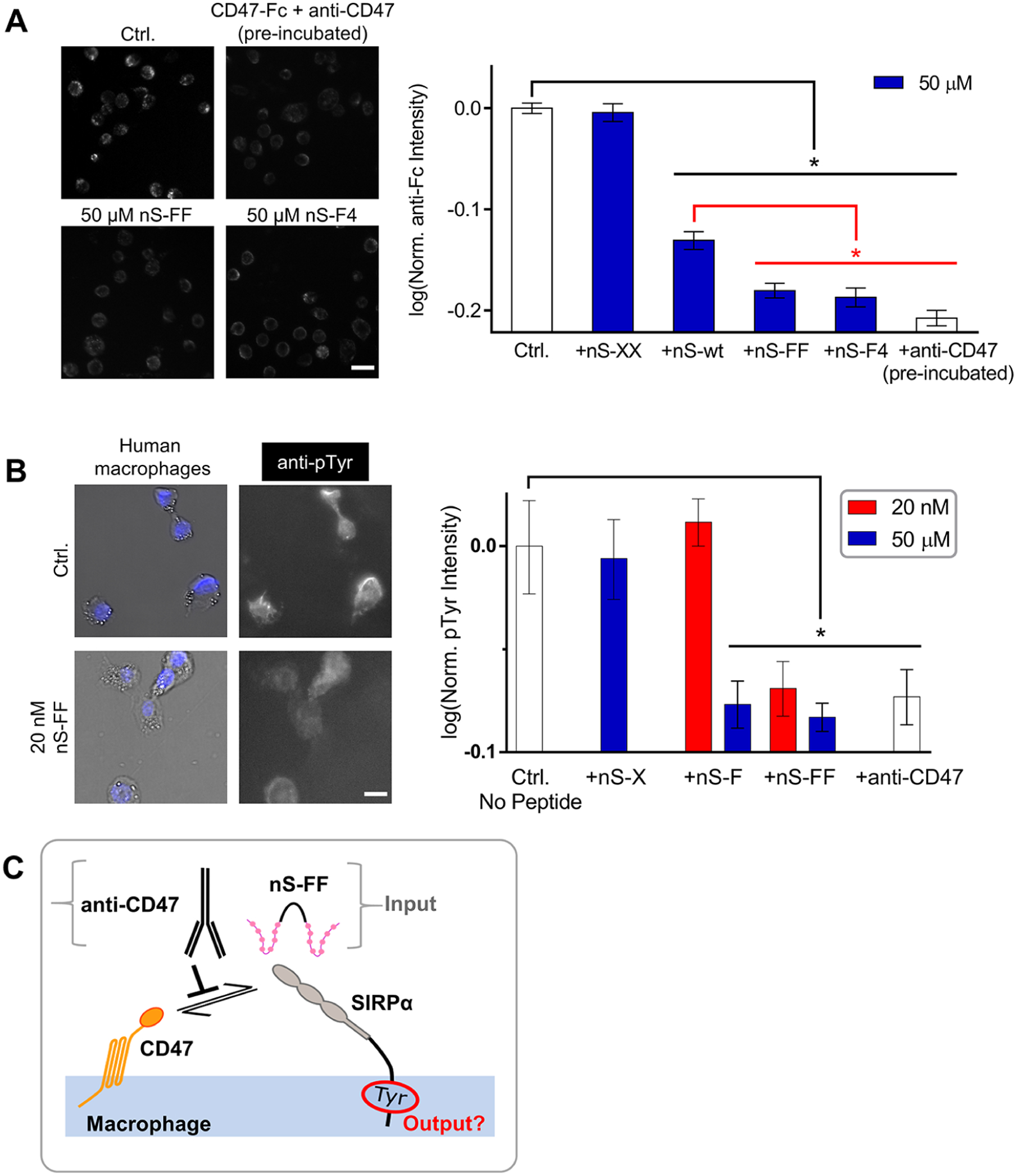
Interactions of nano-Self peptides with macrophages are consistent with SIRPα binding, blocking, and signaling. (A) Representative fluorescence microscopy images of CD47-Fc binding to macrophages as visualized with fluorescent anti-Fc (Ctrl) and also inhibited by nS-wt, nS-FF and nS-F4 peptides but not by scrambled nS-XX. Anti-CD47 and CD47-Fc were incubated together prior to their addition to macrophages. All conditions compared to saturating concentration of CD47-Fc (n = 2 sets of triplicates, mean ± SEM; * denotes p < 0.05; scale bar: 50 μm). (B) Basal levels of pTyr signal are observed in isolated macrophages. The pTyr signal is suppressed by nS-FF even at nM concentration, whereas monovalent nS-F requires μM concentration. This inhibition of phosphorylation is not observed with nS-X (n = 3 ± SEM; * denotes p < 0.05 relative to control; scale bar: 25 μm). (C) Schematic of nS peptides and anti-CD47 antagonizing the cis macrophage checkpoint and thereby suppressing pTyr.
