Figure 1.
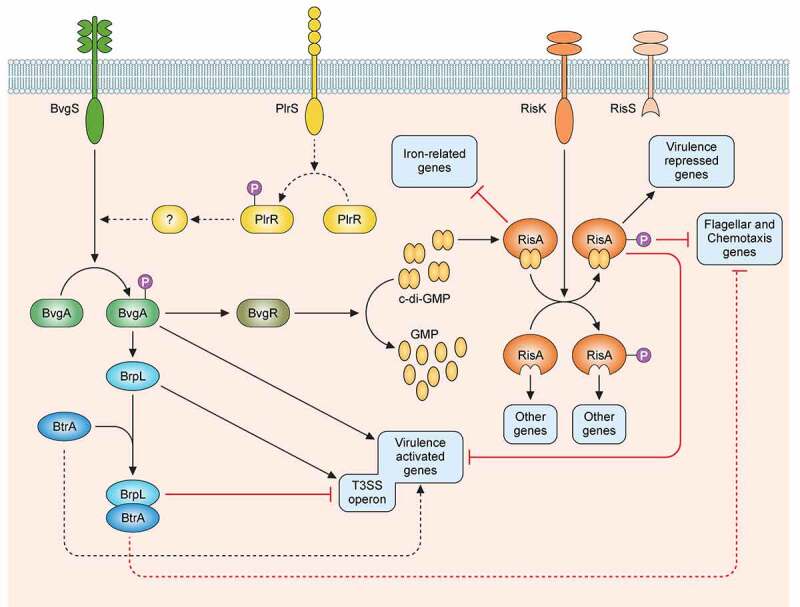
Regulatory network of the virulence factors in B. pertussis. BvgS is a sensor-kinase that is active by default at a temperature of 37°C. Its autophosphorylation is followed by the phosphorylation of its cognate response regulator BvgA. Phosphorylated BvgA triggers the expression of vir-activated genes (vags). BvgS responds to chemical stimuli such as sulfate or nicotinate ions by switching off the phosphorylation cascade, which increases the expression of vir-repressed genes (vrgs). BvgR is the product of a vag and hydrolyzes c-di-GMP to GMP. c-di-GMP affects the activity of the RisAK two-component system by binding to the response regulator RisA. The sensor-kinase RisS is truncated and nonfunctional in B. pertussis, and the partner of RisA is the sensor-kinase RisK. The targets of RisA depend on its phosphorylation and on the concentration of c-di-GMP. Non-phosphorylated RisA bound to c-di-GMP represses the expression of iron-related genes. With both c-di-GMP and phosphorylation RisA induces the expression of the vrgs and represses that of the vags and of the mobility genes, whereas in the absence of c-di-GMP, RisA induces the expression of other sets of genes depending on its phosphorylation. The sensor-kinase PlrS responds to CO2 by phosphorylating the response regulator PlrR. The regulon of PlrSR is unknown, but one of its target(s) interact(s) directly or indirectly with the BvgAS system in an uncharacterized manner. BrpL is a vag coding for a sigma factor that triggers the expression of the T3SS. BtrA antagonizes the activity of BrpL by titrating it in a BtrA/BrpL complex, which results in the repression of the T3SS and the mobility genes and the overexpression of certain vags. The dotted arrows represent interactions identified in B. bronchiseptica and suspected in B. pertussis.
