Figure 2 .
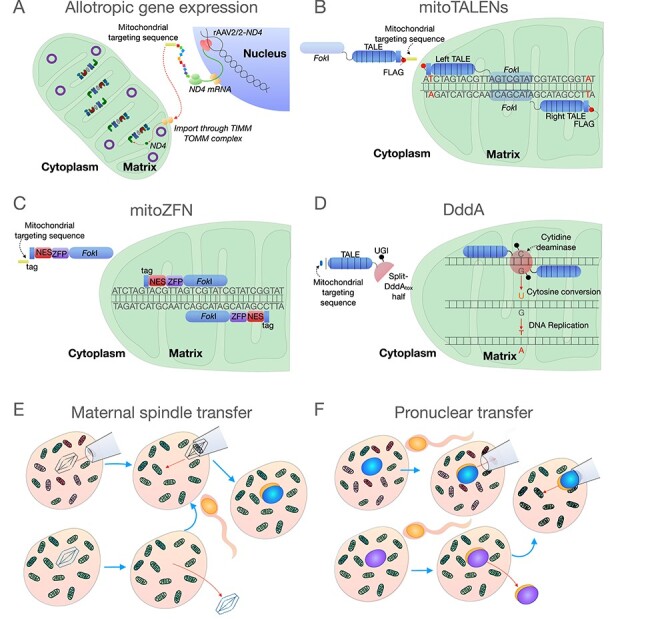
Strategies to rescue and prevent transmission of pathogenic mtDNA variants. (A) Allotropic gene expression has been used to rescue pathogenic variants of Leber’s Hereditary Optic Neuropathy using an adenoviral associated vector containing the MT-ND4 gene (rAAV2/2-ND4) with a mitochondrial targeting sequence. This allows the gene to be transcribed in the nucleus, translated in the cytosol, and later imported into the mitochondria to be incorporated into complex I. In theory, this could be applied to other mtDNA gene variants in the future. (B) MitoTALENs have been used to modulate the heteroplasmy of mtDNA single-nucleotide variants. These nucleases have a mitochondrial targeting sequence which allows them to be imported into the mitochondria. MitoTALENs work in pairs (left and right) which bind to mtDNA variant sequences, bringing the FokI nucleases into close proximity, which leads to cleavage of variant mtDNA molecules and subsequent degradation. (C) mitoZFNs, like mitoTALENS, have been used to modulate mtDNA variant heteroplasmy. The Zinc finger nuclease construct also has a mitochondrial targeting sequence for import into the mitochondria. Like with TALENS, the mitoZFNs bind to the mtDNA variant based on sequence-specificity, bringing the two FokI nucleases together and leading to cleavage of the mtDNA variant, which is degraded. (D) Ddda have been used to revert an mtDNA single-nucleotide variant to its wild-type state via cytosine conversion. A mitochondrial targeting sequence is used to import the construct into the mitochondria, where two TALE sequences locate the two halves of the DddA over the variant site. The Ddda enzyme converts cytosine to uracil. When the DNA is replicated this U-G pair is then converted to a T-A pair. (E, F) Mitochondrial donation for women with pathogenic mtDNA variants. Mitochondrial donation involves the transfer of the nuclear DNA from an zygote (E) or oocyte (F) from a woman with a pathogenic mtDNA variant into an enucleated, recipient donor zygote or oocyte with healthy mtDNA in order prevent the transmission of the pathogenetic mtDNA variant to the offspring. In pronuclear transfer (E) both donor and patient oocytes are fertilized and allowed to progress into the zygote, then the pronuclei of the donor zygote is removed and replaced by the pronuclei from the patient. In maternal spindle transfer (F), the metaphase II meiotic spindle of a donor oocyte is removed and replaced by the spindle from a mature oocyte of the patient followed by fertilization and development.
