Figure 4.
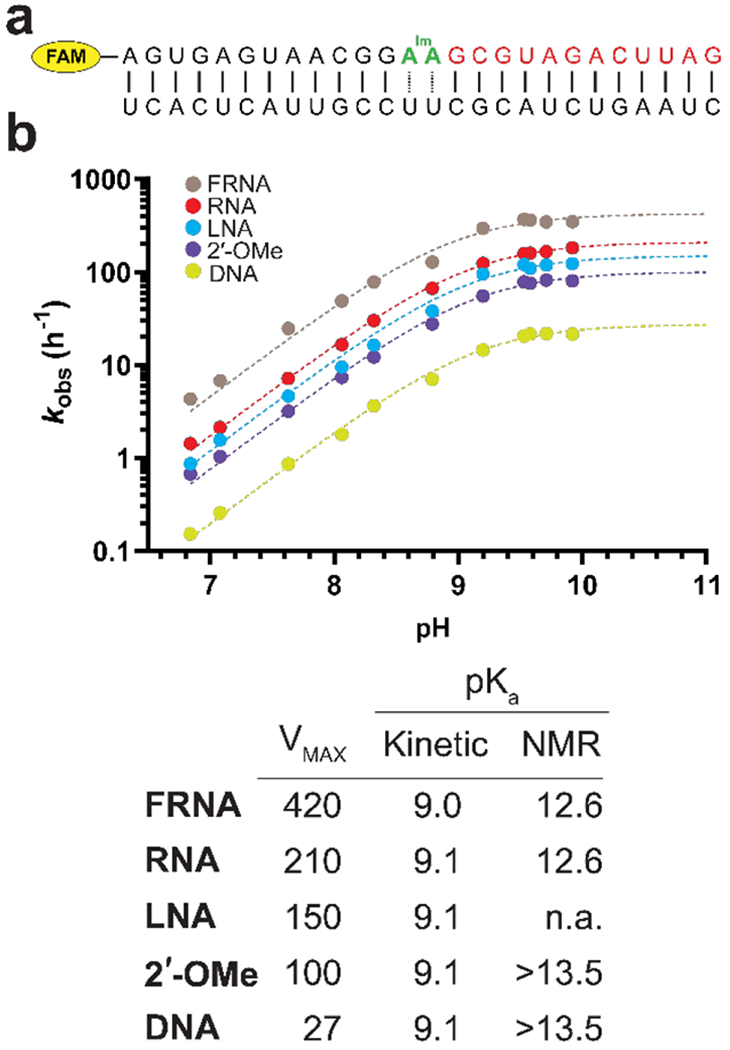
Determination of the 3′-OH pKa for five modified nucleotides. (A) The construct used for the primer extension experiments. The oligonucleotide in red is a downstream binder which pre-organizes the duplex[16] and increases the affinity of the template for the imidazolium bridged intermediate. (B) pH-rate profiles for primer extension with primers containing five distinct 3′-terminal residues. Each reaction was carried out in triplicate, and only the mean values were used to fit the data. The A*A imidazolium bridged dinucleotide and 200 mM Mg2+ were used in the primer extension reactions. The table contains the pKa and VMAX, determined from the pH-rate profiles. The right most column shows nucleoside pKa values determined by 1H NMR in the absence of Mg2+.
