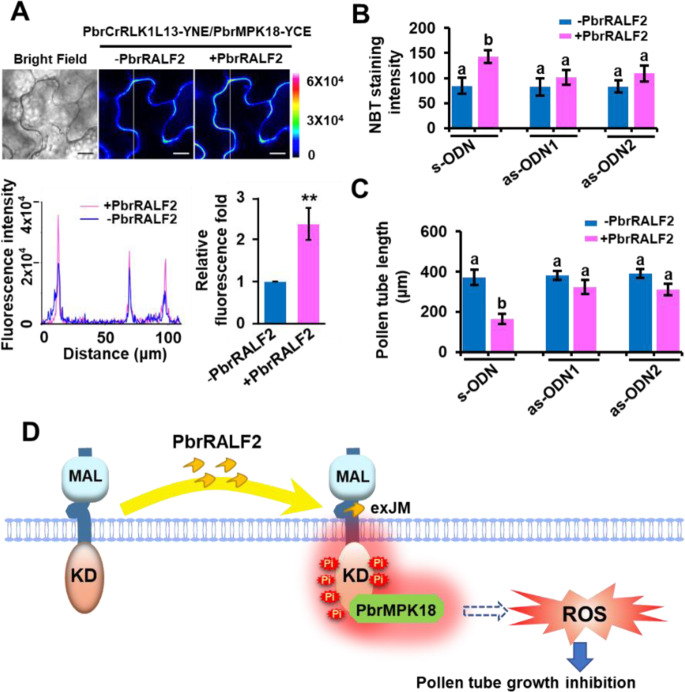
Fig. 6. PbrRALF2 enhances the PbrCrRLK1L13-PbrMPK18 interaction, and PbrMPK18 is required for PbrRALF2 signaling.
A Interaction of PbrCrRLK1L13 with PbrMPK18 analyzed by BiFC in N. benthamiana epidermal cells. The interaction levels of PbrCrRLK1L13 and PbrMPK18 were significantly enhanced by 1 μM PbrRALF2. (** represents P < 0.01; Student’s t test). Data points are presented as the means ± s.e.m. Scale bar = 20 μm. B PbrMPK18 is required for PbrRALF2-induced ROS accumulation. PbrMPK18 was knocked down by as-ODN1 and as-ODN2, with s-ODN as a negative control. PbrRALF2 (1 μM) was used to treat the pollen tubes. ROS accumulation was tested by NBT staining. Thirty-five pollen tubes were measured in each of three independent experiments. Different letters indicate significant differences, as determined by two-way ANOVA. C Inhibition of pollen tube growth was not sensitive to PbrRALF2 when PbrMPK18 was knocked down by as-ODN in pollen tubes. Thirty-five pollen tubes were measured in each of three independent experiments. Different letters indicate significant differences, as determined by two-way ANOVA. D Proposed model for PbrRALF2 perception in pollen tubes of pear. After PbrRALF2 binds to the exJM region of PbrCrRLK1L13, phosphorylation of PbrCrRLK1L13 is enhanced. Under the mediation of PbrMPK18, phosphorylated PbrCrRLK1L13 increases ROS production in pollen tubes. Excessive ROS production eventually inhibits the growth of pollen tubes