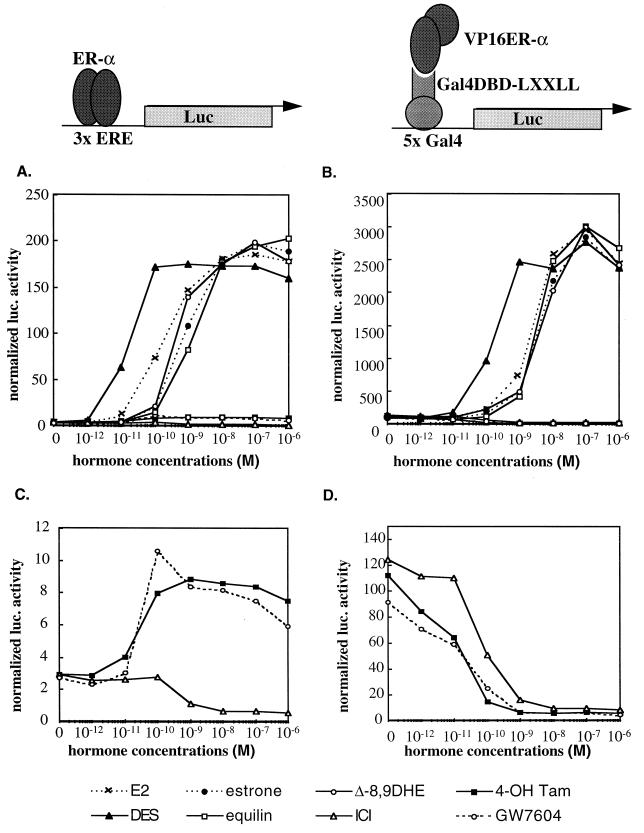
FIG. 2.
The interaction between LXXLL-containing peptides and ER occurs only in the presence of receptor agonists. The LXXLL-containing ER4 peptide sequence was fused to Gal4DBD, while the full-length ERα was expressed as a VP16 transactivation domain fusion protein. The interaction between ER4 peptide and ERα was assessed by using the 5×Gal4Luc3 reporter gene (B and D). The ability of different ER ligands to facilitate LXXLL peptide-ERα interactions was compared to the ability of these ligands to induce ER-mediated transactivation, as assayed by using the 3×ERE-TATA-Luc reporter (A and C). HepG2 cells were transiently transfected with the ERα expression vector (pRST7ERα) and its reporter 3×ERE-TATA-Luc construct (A and C) or Gal4DBD-ER4, pVP16-ERα, and 5×Gal4Luc3 (B and D) and treated with different ER ligands as indicated in the key. Luciferase (Luc) activity was normalized to the activity of the cotransfected pCMVβgal plasmid. E2, 17β-estradiol; 4-OH Tam, 4-hydroxytamoxifen; ICI, ICI 182,780; DES, diethylstilbesterol; Δ-8,9DHE, delta-8,9-dehydroestrone.