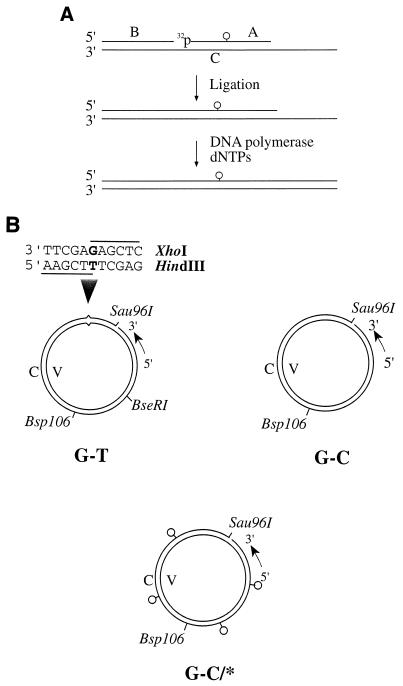
FIG. 1.
DNA substrates. (A) Construction of a 50-mer oligonucleotide containing carcinogen adducts. A 32P-labeled B[a]PDE-containing 11-mer (oligonucleotide A [5′-CCATCG*CTACC-3′]) was ligated with oligonucleotide B (5′-GACTACGTACTGTTACGGCT-3′) after they were annealed to a complementary 50-mer (oligomer C [5′-GCAGATCTGGCCTGATTGCGGTAGCGATGGAGCCGTAACAGTACGTAGTC-3′). The ligated oligomer was then elongated to 50 nucleotides by using oligonucleotide C as the template. The 50-mer homoduplex (G-C) and heteroduplex (A-C) were also constructed by using oligonucleotides 5′-CCATCGCTACC-3′ and 5′-CCATCACTACC-3′, respectively, as oligonucleotide A. dNTPs, deoxynucleoside triphosphates. (B) Circular DNA substrates. Each 6,440-bp circular substrate contained a strand break at the Sau96I site in the complementary (C) strand. Substrate G-T was a heteroduplex containing a single G-T mismatch, substrate G-C was a homoduplex, and substrate G-C/* was a homoduplex modified by chemical carcinogens in the complementary strand. The small circles represent carcinogen-DNA adducts. Several restriction sites relevant to this study are also shown.