Abstract
Developing immunosuppressive therapies for autoimmune diseases comes with a caveat that immunosuppression may promote the risk of developing other conditions or diseases. We have previously shown that biolistic delivery of an expression construct encoding inducible HSP70 (HSP70i) with one amino acid modification in the dendritic cell (DC) activating moiety 435–445 (HSP70iQ435A) to mouse skin resulted in significant immunosuppressive activity of autoimmune vitiligo, associated with fewer tissue infiltrating T cells. To prepare HSP70iQ435A as a potential therapeutic for autoimmune vitiligo, in this study we evaluated whether and how biolistic delivery of HSP70iQ435A in mice affects anti-tumor responses. We found that HSP70iQ435A in fact supports anti-tumor responses in melanoma-challenged C57BL/6 mice. Biolistic delivery of the HSP70iQ435A-encoding construct to mice elicited significant anti-HSP70 titers, and anti-HSP70 IgG and IgM antibodies recognize surface-expressed and cytoplasmic HSP70i in human and mouse melanoma cells. A peptide scan revealed that the anti-HSP70 antibodies recognize a specific C-terminal motif within the HSP70i protein. The antibodies elicited surface CD107A expression among mouse NK cells, representative of antibody-mediated cellular cytotoxicity (ADCC), supporting the concept, that HSP70iQ435A-encoding DNA elicits a humoral response to the stress protein expressed selectively on the surface of melanoma cells. Thus, besides limiting autoimmunity and inflammation, HSP70iQ435A elicits humoral responses that limit tumor growth and may be used in conjunction with immune checkpoint inhibitors to not only control tumor but to also limit adverse events following tumor immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-021-01229-x.
Keywords: Vitiligo, Dendritic cell, Anti-tumor, Melanoma, Gene gun, ADCC
Introduction
Heat shock proteins (HSP) are a family of conserved molecular chaperones. Among this family of proteins is the 70 kDa protein named HSP70 that accounts for 1–2% of total cellular protein (Katschinski 2004). Heat shock proteins play an important role in protein folding, and prevent protein aggregation (Beckmann et al. 1990). When a cell is stressed, the inducible HSP70 isoform also known as HSPA1A/A1B is overexpressed to maintain cellular homeostasis and protect the cell from apoptosis (Beere 2004; Jäättelä 1999). Cellular stress is a common feature in many diseases, and HSP70i frequently plays a crucial role in disease pathology, with examples ranging from Parkinson’s and Alzheimer’s disease (Witt 2010), viral infections (Kim and Oglesbee 2012; Young 1990), and autoimmune diseases (Mansilla et al. 2014, 2012; Mosenson et al. 2012) to cancers (Sherman and Gabai 2015). In cancer, the location of HSP70i can dictate its participation in pro-tumoral or anti-tumoral activities (Shevtsov et al. 2018). When present intracellularly, HSP70i functions as a chaperone and protects tumor cells from apoptosis, while promoting tumor cell migration and proliferation (Barnes et al. 2001; Kasioumi et al. 2019). However, when HSP70i is present on the surface of a cell or is secreted into the extracellular milieu, it represents a danger signal. Extracellular HSP70i functions as a “chaperokine” (Asea 2005) by presenting chaperoned tumor peptides to dendritic cells (Noessner et al. 2002) and inducing tumor cell chemokine secretion resulting in anti-tumor responses (Chen et al. 2009). Therefore, membrane HSP70i can be exploited as a target for cancer therapeutics.
We showed that wildtype HSP70i is central to autoimmune-mediated skin depigmentation, also known as vitiligo (Mosenson et al. 2012; Denman et al. 2008). Biolistic delivery of HSP70i DNA to mice resulted in activation of pro-inflammatory DCs and enhanced skin infiltration by cytotoxic T cells. Introducing a single amino acid modification into the protein resulted in HSP70iQ435A, a variant with remarkable immunosuppressive properties. The variant HSP70iQ435A effectively tempered depigmentation in spontaneous mouse models of the disease (Mosenson et al. 2013). Biolistic delivery of plasmid DNA encoding this mutant isoform resulted in tolerized DCs and fewer skin-infiltrating cytotoxic T cells. Tolerogenic DCs are capable of inducing T cell anergy and can generate and activate regulatory T cells, thereby reducing the cytotoxic activity of T cells (Domogalla et al. 2017; Iberg and Hawiger 2020). Hence, HSP70iQ435A can serve as a promising treatment candidate for vitiligo and indeed, this was further confirmed in a swine model of progressing vitiligo (Henning et al. 2018). In addition, the DC tolerizing phenotype of HSP70iQ435A is a promising therapeutic approach for conditions that involve excessive inflammation (Audiger et al. 2017). However, suppressing immune activation towards these self-antigens brings an increased risk of promoting tumor growth in treated patients. Tissue-resident memory (TRM) CD8+ T cells are found in vitiligo skin (Boniface et al. 2018) and promote anti-melanoma immune activity (Park et al. 2019) with the cooperation of DCs (Menares et al. 2019). These findings suggest that modulating skin-resident immune cells will influence tumor control. Therefore, it is critical to understand whether HSP70iQ435A DNA treatment alters tumor growth dynamics.
In this study, we measured anti-tumor protection offered by HSP70iQ435A-encoding plasmid DNA in mice. We also evaluated expression of HSP70i in control and malignant tissue samples, and established increased expression by tumor cells under stress. We further tested whether serum antibodies in HSP70iQ435A-treated animals can recognize surface-expressed HSP70, and measured the involvement of IgG and IgM responses to the molecule, as well as NK cell degranulation by serum antibodies to the heat shock protein. To identify the epitope recognized by serum antibodies, a peptide scan was performed, and substitution of each amino acid in the cognate peptide was performed to identify a peptide optimally recognized by serum antibodies to the molecule, with possible implications for anti-tumor vaccination. This work can help us understand whether HSP70iQ435A-encoding plasmid DNA can safely serve to curb autoimmune responses and side effects of targeted therapy for melanoma.
Methods
Immunohistology
Human skin biopsies from healthy foreskin, skin from lentigo maligna patients, and metastatic tumors from melanoma patients were obtained as otherwise discarded tissue samples with approval from the Loyola University Medical Center IRB, adherent to the principles outlined in the accord of Helsinki (Association 2013). Fresh tissues (n = 3 for each group) were snap-frozen, embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). Eight micrometers thick sections were cut in a cryostat (Leica Biosystems, USA) and fixed in cold acetone (Thermo Fisher Scientific, Waltham, MA, USA) before indirect immunoperoxidase staining was performed as described previously (Mosenson et al. 2013). Briefly, aspecific staining was blocked by preincubation with 10% normal human serum in PBS. Tissue sections were then exposed to mouse anti-HSP70/HSP72 IgG1 (clone C92F3A-5) monoclonal antibody (Enzo Life Sciences, Farmingdale, NY, USA) SPA810 (1:50) before adding peroxidase-labeled IgG1 anti-mouse antibody (1:200 SouthernBiotech, Birmingham, AL, USA). In separate stainings, mouse tumor cryosections were subjected to peroxidase horseradish peroxidase-labeled rabbit anti-mouse IgG antiserum (1:200 SouthernBiotech). Commercially prepared 3-Amino-9-Ethylcarbazole (AEC) detection solution (Abcam, Cambridge, MA, USA) was used to develop the staining. Tissues were subjected to Harris hematoxylin (Sigma-Aldrich, St. Louis, MO, USA) counterstaining as warranted prior to coverslipping in glycergel (Dako). Human tissue stainings were imaged (two regions of interest (ROI) per tissue) using an Olympus BX51 (Center Valley, PA) microscope, and two investigators independently scored the resulting staining intensities, using modified scoring criteria (Chlipala et al. 2020), as 0—no staining, 1—minimal staining, 2—some staining, 3—-moderate staining, and 4—intense staining. Mouse tumor stainings were evaluated by 3 investigators for images obtained using a Revolve R4 microscope (Echo, San Diego, CA) under the same evaluation criteria.
Biolistic delivery
Biolistic delivery was used to introduce expression plasmids into the skin of mice and measure the effects of the encoded gene products in vivo. Full-length human wildtype HSP70i and human mutant HSP70i plasmid DNA were subcloned from c-terminal GFP TOPO expression vector into pUMVC3 (a kind gift from the University of Michigan vector core). Empty vector (EV) plasmid pUMVC3 was used as indicated to serve as negative control DNA. Biolistic delivery of these plasmids was performed in C57BL/6 J wildtype mice (Jackson Laboratories, Bar Harbor, ME) by gene gun as described previously (Mosenson et al. 2013). Briefly, endotoxin-free plasmid DNA was precipitated onto spermidine-coated gold nanoparticulate beads (Sigma-Aldrich) that were then layered onto silicone tubing (Bio-Rad, Hercules, CA, USA). The resulting tubing was dried under nitrogen gas and cut into bullets, which were maintained under vacuum pressure and used within 14 days. Mice (n = 6 per group) were prepared by shaving abdominal hair and bullets were introduced into the skin under isoflurane anesthesia (E-Z Euthanex gas chamber, E-Z Systems Corp.) with a Helios Gene Gun (Bio-Rad). Mice were treated for four consecutive weeks with 5 µg/week of DNA. All animal procedures were performed under Institutional Animal Care and Use Committee (IACUC) approval at Loyola and Northwestern Universities and in accordance with the Guide for the Care and Use of Laboratory Animals (Albus 2012).
Melanoma tumor challenge
To evaluate the consequences of overexpressing the wildtype, immune-activating HSP70i isoform or its Q435A modified variant, mice exposed to either plasmid via biolistic delivery were later tumor challenged. To this end, 1 × 105 B16.F10 cells (ATCC, Manassas, VA) or 5 × 105 Yummer1.7 cells (Wang et al. 2017) were harvested in logarithmic growth phase and 100 µL of cells suspended in phosphate-buffered saline (PBS, Thermo Fisher Scientific), were injected subcutaneously into both flanks of gene gun–treated mice. As relevant, the resulting tumor growth was measured by evaluating tumor volumes, recorded daily from day 5 onwards, using calipers. The standard formula (π/6) × Length × Width × Height was used to calculate tumor volume. All animal procedures were performed under IACUC approval and in accordance with the Institutional Animal Care and Use Committee guidelines at Loyola and Northwestern Universities.
Measuring anti-HSP70 titers
Blood from gene gun–treated mice was collected by cardiac puncture at euthanasia and transferred into microtainer tubes (BD, Franklin Lakes, NJ, USA). Serum was removed and stored at − 20 °C until use. Serum antibody titers were determined using the Anti-HSP70 IgG/A/M ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA) with slight modifications to the protocol. Briefly, the standard curve for this ELISA was developed using the SPA810 antibody. To determine total anti-HSP70 IgG titers, peroxidase (HRP)–conjugated polyclonal goat anti-mouse IgG horseradish antibodies (SouthernBiotech, Birmingham, AL, USA) were used. To detect subclass-specific titers, HRP-conjugated anti-mouse IgG1, IgG2a, IgG2b, IgG2c, or IgG3 antibodies (all from SouthernBiotech) were used. The ELISAs were developed using 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate and absorbance was measured at 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
Cellular heat shock
To evaluate overexpression of HSP70i by tumor cells under stress, as experienced by tumor cells in vivo, cultured melanoma cells were exposed to a brief heat shock. B16.F10 mouse melanoma cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, Bio-Techne, Minneapolis, MN, USA) and 1% antibiotic/antimycotic (AA, Thermo Fisher Scientific); 624.38 human melanoma cells were maintained in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% FBS and 1% AA. Cells were grown overnight in 60 mm dishes (Corning Inc., Corning, NY, USA). The next day, culture medium was replaced with 42 °C pre-warmed medium and the dishes were covered with parafilm and placed in a circulating water bath (Thermo Fisher Scientific) at 42 °C for 1 h (heat shocked). Control dishes received 37 °C pre-warmed medium and were left at 37 °C. After 1 h, culture medium was replaced with 37 °C pre-warmed medium in both the heat-shocked and non-heat-shocked dishes. The dishes were incubated overnight for 16–18 h at 37 °C to leave time for de novo protein expression in vitro, before harvesting the cells.
Immunoblotting
Cells were lysed using radioimmunoprecipitation (RIPA) buffer (Sigma-Aldrich) with a cocktail of protease/phosphatase inhibitors (Thermo Fisher Scientific) for 30 min on ice. Debris was pelleted and lysates were combined with Laemmli sample buffer (Bio-Rad) and 2-Mercaptoethanol (Sigma-Aldrich), and denatured by heating at 95 °C for 10 min before loading onto a 4–20% Mini-PROTEAN TGX gel (Bio-Rad). Electrophoresed proteins were transferred to methanol-activated polyvinylidene difluoride (PVDF) membranes (Sigma-Aldrich). Membranes were blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich) in PBS (pH: 7.4, Thermo Fisher Scientific) with 0.1% Tween-20 (Sigma-Aldrich) (PBS-T) for 1 h and incubated with sera from EV- or HSP70iQ435A-treated mice (1:100), SPA810 (1:1000), SPA811 (1:1000), or β-actin (1:1000) (BioLegend, San Diego, CA, USA) overnight at 4 °C. Membranes were then incubated with HRP-conjugated horse anti-mouse IgG secondary antibody (1:10,000) (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature and then developed using AEC solution (Abcam) for 30 min at room temperature. Membranes were scanned and converted to grayscale images using Photoshop (Adobe, San Jose, CA, USA). Bands were quantified using ImageJ (Schneider et al. 2012), normalized to β-actin, and then normalized to non-heat-shocked cells.
Surface HSP70 expression and FACS analysis
624.38 cells were incubated with PBS or pooled sera (1:100 dilution in PBS, from 3 gene gun treated mice) or SPA810 antibody (1:100 dilution in PBS) for 30 min, before cells were incubated with FITC-labeled goat anti-mouse (SouthernBiotech, 1:200 dilution) along with Live/Dead Fixable Near-IR (Thermo Fisher Scientific) dye. Data were acquired on FACSymphony flow cytometer (BD Biosciences) and analyzed using FlowJo v10.6.1 (FlowJo LLC, Ashland, OR, USA). HSP70 surface expression was detected on live, single cells. For immune monitoring of Yummer1.7 tumors, homogenates were subjected to a panel of antibodies including Live/Dead staining as above, BB515-labeled 30-F11 to CD45, APC Cy7-labeled PK136 to NK1.1, APC-R700 labeled N418 to CD11c, and BUV737-labeled M1/70 to CD11b (all from BD BioSciences, Franklin Lakes, NJ), and subjected to FACS analysis as above.
Epitope mapping
Epitope mapping was performed using a peptide microarray (PEPperPRINT GmbH, Heidelberg, Germany). Briefly, the C-terminus of human HSP70i amino acid sequences were elongated with neutral GSGSGSG linkers at the C- and N-terminus to avoid truncated peptides. The elongated sequence was translated into 15 amino acid peptides with an interpeptide overlap of 14 amino acids. A peptide microarray was developed by printing the peptide sequences in duplicate with HA (YPYDVPDYAG) peptides serving as controls. The microarray was blocked with a blocking buffer (Rockland Immunochemicals, Inc., Limerick, PA, USA) for 30 min prior to incubating it with pooled sera from mutant HSP70 treated animals for 16 h at 4 °C with constant shaking at 140 rpm. Then, the microarray was incubated with goat anti-mouse IgG (Fc) DyLight680 or mouse monoclonal anti-HA (12CA5) DyLight800 secondary antibodies. Fluorescence was detected using the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Image analysis was performed, and fluorescence intensities were calculated using the PepSlide Analyzer (PEPperPRINT GmbH).
Epitope substitution scan
Epitope substitution scanning was performed similar to the epitope mapping procedure described above, apart from the microarray and the secondary antibody. The microarray was printed with the wildtype sequence SGLYQGAGGPGPGGF along with sequences where each amino acid in the wildtype sequences was substituted with 20 other amino acids. The resulting peptides were printed in triplicate, incubated with serum from HSP70iQ435A exposed mice, and responses were detected using the DyLight680-labeled goat anti-mouse IgG (H + L) secondary antibody. Fluorescence detection and analysis were performed as described under above epitope mapping.
Degranulation assay
HSP70 protein–coated plates (Enzo Life Sciences) were incubated with sera from EV and HSP70iQ435A-treated mice before incubating the plate with 2 × 106 C57BL/6 total splenocytes in presence of monensin (BioLegend) and CD107a-PE/Cy7 antibody (clone:1D4B, BioLegend) for 5 h at 37 °C. The splenocytes were collected and exposed to Fc-block (TruStain FcX anti-mouse CD16/CD32, clone: 93), then stained with antibodies to mouse CD45-BB515 (clone: 30-F11), NK1.1-BV711 (clone: PK136), CD11b-BUV737 (M1/70) (all from BioLegend), and Live/Dead Fixable Near-IR (Thermo Fisher Scientific) dye. Data were acquired on FACSymphony flow cytometer (BD Biosciences) and analyzed using FlowJo v10.6.1 (FlowJo LLC, Ashland, OR, USA). To identify cytotoxic activity on NK cells, the percentage of CD107a+ cells in NK1.1+ cells were gated from live, single CD45+ cells.
Statistical analysis
Statistics was performed using Prism software (version 9, GraphPad Software, San Diego, CA, USA). For mouse experiments, group sizes of 6 mice per group or above were included for 80% power to detect statistically significant differences at α = 0.05. To compare tumor measurements among groups, the area under the curve (AUC) for each group was calculated and divided by the total number of days of available data minus 1. One-way ANOVA was then used to compare the time-adjusted AUC among groups, or tissue staining among groups. To compare outcomes in experiments involving two experimental groups, Student’s test was used. When comparing three or more groups, a one- or two-way ANOVA was followed by Tukey’s post-test to identify differences between individual groups, applying Bonferroni’s correction for multiple comparisons. For mouse IgG staining of tumor sections, one-way ANOVA was followed by Dunnett’s post hoc test for multiple comparisons.
Results
HSP70i is overexpressed in human melanoma tissues
Hypoxia and oxidative stress are associated with malignant transformation of melanocytes (Bedogni and Powell 2009; Bisevac et al. 2018) suggesting a role of heat shock proteins in melanoma growth and progression. We sought to determine the expression of HSP70i representing different stages of malignant transformation in human melanoma tissues and compare the protein expression to healthy control skin. Indeed, compared to the healthy skin (Fig. 1a, bottom panel) as well as lentigo maligna tissue (Fig. 1a, bottom panel), immunostaining analysis revealed an overexpression of HSP70i in metastatic melanoma tissue (Fig. 1a, bottom panel). The secondary antibody only controls were clean for all tissue types (Fig. 1a, top panel). Furthermore, the staining intensity was quantified (Fig. 1b) in a blinded fashion, following a scale from 0 to 3 scale representing non-expressing to high expressing tissue. A full panel of stainings from 3 tissues per group is shown in Fig. S1. A significantly high increase in mean score (2.8) was observed comparing melanoma to lentigo maligna and healthy controls (P < 0.01 and P < 0.001, respectively), confirming the overexpression of HSP70i in metastatic melanoma tissues. Our results are consistent with existing findings that HSP70i is overexpressed in melanoma tumors (Budina-Kolomets et al. 2016; Lazaris et al. 1995).
Fig. 1.
HSP70i is overexpressed in human melanoma skin. a Representative images of healthy control, lentigo maligna (benign melanoma), and metastatic melanoma tissues showing overexpression of HSP70i (red). Tissues treated with secondary antibody only were used as a staining control. Scale bar: 50 µm. b Quantification of staining intensity (n = 3 per group). Mean ± SD are shown. Asterisks denote statistical significance determined by unpaired t-test: **P < 0.01 and **** P < 0.0001
HSP70iQ435A DNA treatment elicits anti-tumor responses
Biolistic delivery of plasmids into the skin of mice enables the expression of the encoded gene products in vivo (Yang et al. 1996). We previously showed that biolistic delivery of HSP70iQ435A DNA halted depigmentation in mice, tolerized DCs, and hence reduced T cell activation (Mosenson et al. 2013). To understand the consequence of this immune suppression in the context of melanoma, we tested the ability of HSP70iQ435A DNA to prevent tumor growth in C57BL/6 mice. B16.F10 mouse melanoma cells were subcutaneously injected in the flanks of mice that had received either wildtype or mutant HSP70i DNA, or no pretreatment, and tumor growth was assessed over time. While the wildtype HSP70i DNA–treated mice (Fig. 2, green line) showed significant tumor control (P < 0.01) compared to the no treatment group (Fig. 2, red line), notably, the HSP70iQ435A DNA–treated mice (Fig. 2a, blue line) also showed significant tumor control (P < 0.01) compared to the no treatment group. This result is interesting as mice that received HSP70iQ435A DNA treatment would have reduced T cell activation because of DC tolerization (Mosenson et al. 2013). Significant control of tumor growth in this group suggested the involvement of another mechanism in controlling tumor growth. We showed that biolistic delivery of HSP70iQ435A DNA in mice induced a humoral response to the C-terminus of HSP70i (Mosenson et al. 2013). We also found that tumors from mice pretreated with HSP70i or HSP70iQ435A-encoding DNA display significantly enhanced binding of mouse IgG antibodies, as shown in Fig. S2. However, the treatment does not alter CD11b/CD11c ratios in the tumor and DNA skin delivery may not affect DC physiology within the tumor (Fig. S3). Overexpression of HSP70i by tumor cells might sensitize the tumor to complement-dependent cytotoxicity (CDC) or antibody-dependent cellular cytotoxicity (ADCC) after cells are opsonized by anti-HSP70-reactive antibodies, as exploited by others in passive immunization therapy in mice (Stangl et al. 2011). We thus hypothesized that anti-HSP70 antibodies, as a consequence of biolistic delivery, may recognize HSP70 on the tumors and contribute to anti-tumor activity by ADCC or CDC.
Fig. 2.

HSP70iQ435A DNA treatment elicits an anti-tumor response. Mice (n = 6 per group) received the indicated treatments 5 times every 6 days. One week after final treatment, 1 × 105 B16.F10 cells were subcutaneously injected into both flanks. Tumors were mea-sured daily starting day 5 post tumor challenge. Mean ± SEM are shown. Asterisks denote statistical significance determined by one-way ANOVA of time-adjusted area under the curve (AUC): ** P < 0.01
Anti-HSP70 antibodies in sera can recognize HSP70i in melanoma cell lines
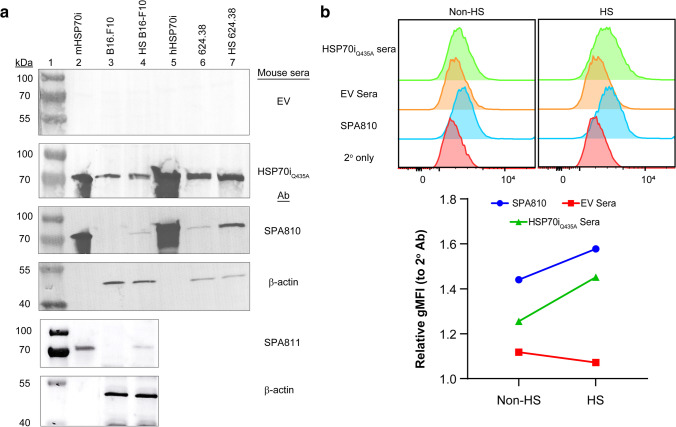
For anti-HSP70 antibodies to recognize HSP70i and kill tumor cells, HSP70i should be overexpressed and be present on the surface of melanoma cells. We first evaluated whether anti-HSP70 antibodies in the sera of HSP70iQ435A DNA–treated mice could recognize HSP70i in melanoma cell lines via immunoblotting using heat-shocked and non-heat-shocked melanoma cell lysates. We reasoned that heat shock would cause thermal stress and overexpression of heat shock proteins, including HSP70i. This was confirmed when the lysates were probed with SPA810 antibody that specifically detects HSP70i. Recombinant human and mouse HSP70 proteins were probed as positive controls. Sera from HSP70iQ435A DNA–treated mice specifically recognized the distinct 70–72 kDa bands in the lanes loaded with recombinant mouse (Fig. 3a, lane 2) and human (Fig. 3a, lane 5) HSP70 proteins. Furthermore, sera from HSP70iQ435A DNA–treated mice detected protein expression at 70–72 kDa, compared to sera from EV DNA–treated mice in the cell lysates demonstrating that sera from HSP70iQ435A DNA–treated mice recognize HSP70i. The increase in 70 kD band densities after heat shock relative to β-actin in B16 mouse melanoma cells was 53% or 89% as detected by mouse serum antibodies and SPA810, respectively, and as 48% and 562% in 624.38 human melanoma cells, respectively. By comparison, the increase in band intensity in the same samples of B16 cells detected by the SPA811 antibody amounted to 384% (Fig. 3a, lanes 3, 4, 6, and 7). In every case, there was more HSP70i detected after heat shock, but the actual increase in detection level is more readily quantified by FACS. For antibodies to respond to tumor cells overexpressing the heat shock protein under study, HSP70 should be expressed on the tumor cell surface. Thus, heat-shocked cells were exposed to surface staining and FACS analysis. Surface expression of HSP70 on B16.F10 was shown previously (Stangl et al. 2011; Nimmervoll et al. 2015). Here, we evaluated surface HSP70 expression in 624.38 cells. Baseline surface protein expression was detectable by (Fig. 3b) SPA810 antibody or sera from EV- or HSP70iQ435A DNA–treated mice and the surface expression increased in response to heat shock (Fig. 3b). The geometric mean fluorescence intensity (gMFI) of the histograms (Fig. 3b) revealed a ~ 1.6- and ~ 1.4-fold increase of surface protein expression using SPA810 antibody (blue line) and sera from HSP70iQ435A DNA–treated mice (green line), respectively. Together, these results indicate that antibodies in sera from HSP70iQ435A DNA–treated mice react with overexpressed and surface HSP70i on melanoma cells.
Fig. 3.
Anti-HSP70 antibodies from HSP70iQ435A DNA treatment specifically recognize cytoplasmic and surface HSP70i in human and mouse melanoma. a Representative immunoblots showing detection of HSP70i (~ 72 kDa) with empty vector (EV)– and HSP70iQ435A DNA–treated sera and SPA810 antibody in non-heat-shocked or heat-shocked (HS) B16.F10 and 624.38 cell lysates (lanes 3, 4, 6, and 7 respectively). Purified recombinant mouse (mHSP70i, lane 2) and human (hHSP70i, lane 5) HSP70i were also probed, in addition to β-actin to confirm proper loading. b FACS histograms for surface HSP70i detection (top) and quantification of relative geometric mean fluorescence intensity (gMFI, bottom) in non-heat-shocked and heat-shocked 624.38 human melanoma cells probed with indicated sera and antibody. Representative results from two independent experiments are shown. 2° only: secondary antibody only control
HSP70iQ435A DNA treatment elicits strong anti-HSP70 titers
Our previous data (Denman et al. 2008; Mosenson et al. 2013) and the outcomes above indicate that in response to biolistic delivery of HSP70iQ435A-encoding DNA, a humoral response is generated to HSP70A1A/A1B. To determine the anti-HSP70 antibody titers in the sera of treated mice, we performed ELISA. A significant, 1426-fold (P < 0.01) increase was found in the immunoglobulin G (IgG) anti-HSP70 titers of HSP70iQ435A DNA–treated mice compared to EV DNA–treated mice (Fig. 4a). We next evaluated whether HSP70iQ435A DNA treatment favored the development of specific Ig subclasses that mediate ADCC or CDC. Our results revealed that IgG1 (~ fourfold change), IgG2b (~ 107-fold change), IgG2c (~ 77-fold change), and IgG3 (~ 19.4-fold change) antibodies were significantly higher (P < 0.01 and P < 0.05) in HSP70iQ435A DNA–treated sera compared to EV DNA–treated sera (Fig. 4b). We further observed a trend towards greater IgG2a and IgM in HSP70iQ435A DNA–treated sera (Fig. 4b). This suggests that HSP70iQ435A DNA treatment elicits strong anti-HSP70 titers of IgG1 and IgG2b antibodies that can favor ADCC, and IgM antibodies that can favor CDC (Bruhns and Jönsson 2015; Stewart et al. 2014).
Fig. 4.
HSP70iQ435A DNA treatment elicits strong anti-HSP70 titers. C57BL/6 mice received the indicated treatments 5 times every 6 days. One week after final treatment, blood was collected, and sera were isolated to run an ELISA for anti-HSP70 titers. a Anti-HSP70 IgG titers were higher in HSP70iQ435A DNA–treated animals (n = 10 per group). Mean ± SD are shown. b Absorbance values for different subclasses of anti-HSP70 IgG titers were higher in HSP70iQ435A DNA–treated animals (n = 3 per group). Mean ± SD are shown. Asterisks denote statistical significance determine by unpaired t-test: **P < 0.01 and *P < 0.05
Anti-HSP70 antibodies from HSP70iQ435A DNA treatment recognize a specific epitope on the C-terminus of HSP70i
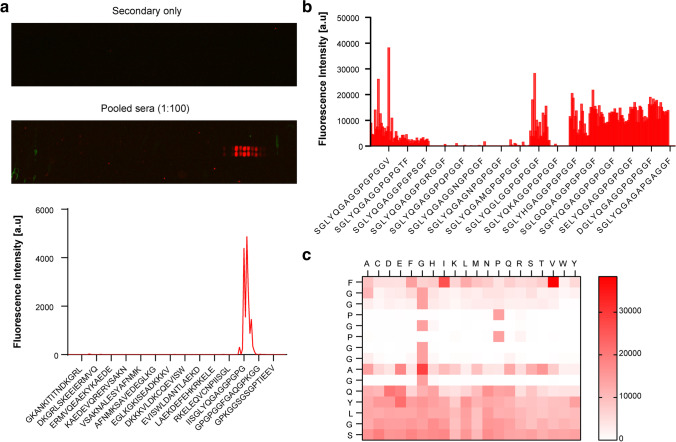
To identify the extracellular epitope(s) recognized by the anti-HSP70 antibodies in the sera of HSP70iQ435A DNA–treated mice, we performed linear epitope scanning analysis. We chose only the C-terminal protein sequence of HSP70 in follow-up to our previous findings (Mosenson et al. 2013). A peptide microarray with overlapping peptides of the C-terminus of HSP70i was prepared and probed with sera from HSP70iQ435A DNA–treated mice. A clear epitope binding response was observed in sequences with the consensus motif YQGAGGPGPG, and the epitope, SGLYQGAGGPGPGGF, showed the highest binding with the anti-HSP70 antibodies (Fig. 5a).
Fig. 5.
Anti-HSP70 antibodies from HSP70iQ435A DNA treatment recognize a specific epitope on the C-terminus of inducible HSP70. a Epitope scanning analysis: image (top) of the peptide microarray with and without the pooled sera and quantification (bottom) of the fluorescence intensity. A 15 amino acid length peptide microarray was printed in duplicate with overlapping regions of the C-terminus of HSP70i and probed with pooled sera from HSP70iQ435A DNA–treated mice. The red spots indicate a positive signal. b Substitution scanning analysis: quantification (left) of the fluorescence intensity image (right) of the peptide microarray probed with and without the pooled sera. Each amino acid in the peptide sequence SGLYQGAGGPGPGGF was substituted with 20 other amino acids and reactivity to anti-HSP70 antibodies was determined. c Heat map depicting fluorescence intensities for each amino acid substitution. The natural sequence of the recognized peptide is shown on the left
An immunogenic epitope from HSP70i may have therapeutic implications that can further be enhanced by replacing individual amino acids with residues that bind to the natural antibodies at higher affinity. To identify resulting, high-affinity peptides, we further investigated the presence of essential and conserved amino acids in the SGLYQGAGGPGPGGF sequence through substitution scanning analysis. Each amino acid in the cognate peptide was substituted, and a peptide microarray was generated and probed with sera from HSP70iQ435A DNA–treated mice. Substitution scanning analysis revealed that exchange of the last amino acid 15F (phenylalanine, a neutral amino acid with a benzyl group) to a hydrophobic amino acid 15 V (valine) improved antibody binding (Fig. 5b). Furthermore, a conserved core motif 6GAGGPGPGG14 was observed and the amino acid positions 6G, 8G, 9G, (glycines, critical to α-helix formation) 10P (proline, a cyclic amino acid), 11G, 12P, and 13G were either essential for antibody binding or at least highly conserved as substitution by other amino acids at these positions resulted in no or diminished antibody binding as seen on the heat map (Fig. 5c). The SGLYQGAGGPGPGGF peptide, injected (IP) into mice bearing B16 tumors, did not significantly boost tumor growth (Figure S4), likely due to the sensitivity of such peptide to proteolytic degradation in vivo. Overall however, these results suggest that anti-HSP70 antibodies recognize a specific epitope in the C-terminus of HSP70i.
Anti-HSP70 antibodies from HSP70iQ435A DNA treatment are capable of triggering natural killer (NK) cell degranulation
Having confirmed that HSP70iQ435A DNA treatment elicits strong anti-HSP70 titers and that these antibodies can recognize overexpressed and surface HSP70i on melanoma cells, we evaluated whether anti-HSP70 antibodies are capable of triggering NK cell degranulation upon recognition of HSP70. We established that tumors from mice pretreated with wildtype or modified HSP70 do not contain greater amounts of NK cells (Fig. S5), but pretreatment may trigger greater NK cell functionality. NK cell degranulation is a measure of antibody-dependent cell cytotoxicity (ADCC) (Morrison et al. 2017) and CD107a is a functional marker to identify NK cell degranulation (Alter et al. 2004). Wells pre-coated with recombinant protein were incubated with sera from EV DNA–treated and HSP70iQ435A DNA–treated mice before adding C57BL/6 splenocytes. CD107a expression by NK cells was detected by flow cytometry. Baseline CD107a-expression among NK cells (5%) in the presence of EV DNA–treated sera was significantly elevated (P < 0.01) among NK cells expressing in the presence of HSP70iQ435A DNA–treated sera, as seen in the dot plots and CD107a quantification (Fig. 6a–b). The data suggests that anti-HSP70 antibodies are capable of triggering NK cell degranulation.
Fig. 6.
Anti-HSP70 antibodies from HSP70iQ435A DNA treatment are capable of triggering NK cell degranulation. Purified HSP70i-coated wells were incubated with pooled sera from HSP70iQ435A and EV DNA treatment. Wells were washed and CD107a-labeled antibody with C57BL/6 splenocytes was added to the wells in the presence of monensin. The wells were incubated at 37 °C for 5 h, after which the cells were collected and stained for FACS. a Dot plots showing a greater percentage of NK expressing CD107a in the presence of antibodies from HSP70iQ435A DNA treatment compared to EV treatment. b Quantification of the percentage of CD107a+ cells. Mean ± SD are shown. Asterisks denote statistical significance determine by unpaired t-test: **P < 0.01
Discussion
An interesting question is how HSP70iQ435A DNA treatment blocked tumor growth in vivo given its ability to tolerize DCs. We hypothesized that anti-HSP70 antibodies generated due to HSP70iQ435A DNA treatment may bind C-terminal HSP70 expressed on the surface of tumor cells, and aid in tumor cell recognition and eventual death. Anti-HSP70 antibodies are generated as a consequence of both HSP70i and HSP70iQ435A DNA biolistic delivery, specifically recognizing the C-terminus of HSP70i (Mosenson et al. 2013). Amino acids 504–617 in the C-terminal region of HSP70 are located on the surface of tumor cells (Botzler et al. 1998) and an antibody to a 14-mer peptide within this region triggered NK cell cytolytic activity towards tumor cells (Multhoff et al. 2001) suggesting that part of the C-terminus of HSP70 is on the tumor surface contributing to tumor cell immune recognition. In line with our hypothesis, HSP70iQ435A DNA treatment elicited antibodies to HSP70 that recognized overexpressed and surface protein in heat-shocked tumor cells. Moreover, the IgG subclasses among these anti-HSP70 antibodies are known to trigger ADCC in mice (Bruhns and Jönsson 2015), and elicited NK cell degranulation here. Epitope analysis revealed that mouse sera responded to amino acids 608–622 in the C-terminus of HSP70i. This peptide sequence is part of the C-terminal region of human HSP70 that is reportedly exposed on the surface of tumor cells (Botzler et al. 1998). A single injection of this peptide into tumor-bearing mice did not significantly enhance tumor growth. Indeed, as small peptides are readily degraded, modifications may be required to stabilize the peptide in vivo and to analyze its blocking activity. The hypoxic nature of tumors and resulting protein misfolding results in the overexpression of heat shock proteins. Among these, HSP70i is secreted by live cells (Mambula et al. 2007). HSP70i is a marker of tumor immunogenicity (Clark and Ménoret 2001) and chaperones peptides for processing by antigen-presenting cells (APC) (Udono and Srivastava 1993). HSP70i thus serves as a damage-associated molecular pattern (DAMP) to induce an immune cascade resulting in anti-tumor T cell responses (Albakova et al. 2020). Therefore, the local T cell responses generated as a consequence of HSP70i overexpression cannot be ruled out in contributing to anti-tumor responses. We thus have outlined a proposed mechanism of action (Fig. 7) by which HSP70iQ435A DNA treatment can block vitiligo and elicit anti-tumor activity. We propose that local delivery of HSP70iQ435A DNA treatment in skin tolerizes local DCs and reprograms skin homing T cells, and generates anti-HSP70 antibodies in the secondary lymphoid organs. These anti-HSP70 antibodies, also found in response to wildtype HSP70i, bind to the overexpressed HSP70i on the surface of melanoma (via the C-terminus) eliciting NK cell degranulation rather than enhanced tumor infiltration by NK cells, resulting in tumor cell killing. In addition, T cells already recruited locally to tumors due to overexpressed HSP70i are primed to target the tumor and aid in tumor killing. Therefore, HSP70iQ435A DNA treatment can not only block vitiligo but can also aid in tumor cell killing.
Fig. 7.
Proposed mechanism of action for HSP70iQ435A DNA treatment to block vitiligo and elicit anti-tumor activity. (1) Local delivery of HSP70iQ435A DNA in the skin leads to (2) reprograming of skin homing effector T cells by skin residing DCs. (3) The DCs then travel through the draining lymph nodes to secondary lymphoid organs where anti-HSP70 antibodies are made. (4) Due to hypoxic conditions in a tumor, HSP70i is overexpressed on the surface of tumor cells which is (5) specifically recognized by the circulating anti-HSP70 antibodies (via the C-terminus) that then (6) results in tumor killing via ADCC. (7) In parallel, the overexpressed HSP70i from tumor cells also recruits T cells that are then primed to target the tumor
Serum antibodies to HSP70 specifically recognized the peptide sequence, SGLYQGAGGPGPGGF, in the C-terminus of HSP70i. Amino acid substitution scanning analysis of this cognate peptide further revealed that a phenylalanine to valine substitution improved anti-HSP70 antibody binding to the HSP70i protein. Therefore, this altered peptide sequence with valine can potentially be used as a peptide-based vaccine (Zhang et al. 2019) to generate high-affinity anti-HSP70 antibodies that could offer anti-tumor protection in humans, though it may be necessary to include one or more sequence modifications to enhance immunogenicity (Guevara-Patiño et al. 2006).
While our results demonstrate that HSP70iQ435A supports anti-tumor responses, it also opens up the feasibility of HSP70iQ435A reducing autoimmune side effects as a consequence of immunotherapies for melanoma. Activation of the immune system by immunotherapies causes immune-related adverse events (irAEs) (Spiers et al. 2019). Skin rashes (Belum et al. 2016) and vitiligo (Hua et al. 2016) are the most commonly reported irAEs with an increased amount of T cell infiltration (Naidoo et al. 2015) in patients receiving immunotherapies for melanoma. Although not life threatening, in patients with pre-existing skin conditions, careful consideration for treatment options is required. While the effect of HSP70iQ435A in skin rash is yet to be investigated, we have evidence that HSP70iQ435A selectively suppresses autoimmune vitiligo without promoting tumor growth of associated melanoma lesions, as demonstrated in Sinclair swine (Henning et al. 2018). The Sinclair swine spontaneously develop melanoma and as they age, the melanoma regresses accompanied by vitiligo around the site of melanoma (Morgan et al. 1996), closely mimicking vitiligo irAEs in melanoma patients receiving immunotherapies. Upon measuring depigmentation and evaluating tumor sizes, we found that HSP70iQ435A halted depigmentation around the melanoma lesions and did not interfere with anti-tumor responses supporting the promising role of HSP70iQ435A to suppress autoimmune side effects associated with melanoma.
In conclusion, this study demonstrates that the DC tolerizing phenotype of HSP70iQ435A does not interfere with tumor growth and instead supports anti-tumor responses, advocating the safety of administering HSP70iQ435A as a treatment for vitiligo, as well as conditions that involve excessive inflammation. Findings from this study also warrant further studies to evaluate the possibility of developing HSP70iQ435A as an anti-tumor treatment in combination with immune check point inhibitors for melanoma.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
These studies were supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) 1R01CA191317 awarded to CLP.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dinesh Jaishankar, Email: jaishank@musc.edu.
I. Caroline Le Poole, Email: caroline.lepoole@northwestern.edu.
References
- Albakova Z, Armeev GA, Kanevskiy LM, Kovalenko EI, Sapozhnikov AM (2020) HSP70 multi-functionality in cancer. Cells 9 (3) [DOI] [PMC free article] [PubMed]
- Albus U. Guide for the care and use of laboratory animals (8th edn) Laboratory Animals. 2012;46(3):267–268. doi: 10.1258/la.2012.150312. [DOI] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The importance of dendritic cells in maintaining immune tolerance. J Immunol. 2017;198(6):2223–2231. doi: 10.4049/jimmunol.1601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones. 2001;6(4):316–325. doi: 10.1379/1466-1268(2001)006<0316:EOIHET>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R, Mizzen L, Welch W. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bedogni B, Powell MB. Hypoxia, melanocytes and melanoma - survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Res. 2009;22(2):166–174. doi: 10.1111/j.1755-148X.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM. ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117(13):2641. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, Lacouture ME. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisevac JP, Djukic M, Stanojevic I, Stevanovic I, Mijuskovic Z, Djuric A, Gobeljic B, Banovic T, Vojvodic D. Association between oxidative stress and melanoma progression. J Med Biochem. 2018;37(1):12–20. doi: 10.1515/jomb-2017-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Jacquemin C, Darrigade A-S, Dessarthe B, Martins C, Boukhedouni N, Vernisse C, Grasseau A, Thiolat D, Rambert J, Lucchese F, Bertolotti A, Ezzedine K, Taieb A, Seneschal J. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Investig Dermatol. 2018;138(2):355–364. doi: 10.1016/j.jid.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3(1):6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P, Jönsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268(1):25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- Budina-Kolomets A, Webster MR, Leu JI, Jennis M, Krepler C, Guerrini A, Kossenkov AV, Xu W, Karakousis G, Schuchter L, Amaravadi RK, Wu H, Yin X, Liu Q, Lu Y, Mills GB, Xu X, George DL, Weeraratna AT, Murphy ME. HSP70 inhibition limits FAK-dependent invasion and enhances the response to melanoma treatment with BRAF inhibitors. Cancer Res. 2016;76(9):2720–2730. doi: 10.1158/0008-5472.CAN-15-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- Chlipala EA, Bendzinski CM, Dorner C, Sartan R, Copeland K, Pearce R, Doherty F, Bolon B. An image analysis solution for quantification and determination of immunohistochemistry staining reproducibility. Appl Immunohistochem Mol Morphol. 2020;28(6):428–436. doi: 10.1097/PAI.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PR, Ménoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6(2):121–125. doi: 10.1379/1466-1268(2001)006<0121:TIHAAM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patiño JA, Le Poole IC. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128(8):2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domogalla MP, Rostan PV, Raker VK, Steinbrink K (2017) Tolerance through education: how tolerogenic dendritic cells shape immunity. Frontiers in Immunology 8 (1764). 10.3389/fimmu.2017.01764 [DOI] [PMC free article] [PubMed]
- Guevara-Patiño JA, Engelhorn ME, Turk MJ, Liu C, Duan F, Rizzuto G, Cohen AD, Merghoub T, Wolchok JD, Houghton AN. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116(5):1382–1390. doi: 10.1172/jci25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning SW, Fernandez MF, Mahon JP, Duff R, Azarafrooz F, Guevara-Patiño JA, Rademaker AW, Salzman AL, Le Poole IC. HSP70i(Q435A)-encoding DNA repigments vitiligo lesions in sinclair swine. J Invest Dermatol. 2018;138(12):2531–2539. doi: 10.1016/j.jid.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, Tomasic G, Soria JC, Champiat S, Texier M, Lanoy E, Robert C. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- Iberg CA, Hawiger D. Natural and induced tolerogenic dendritic cells. J Immunol. 2020;204:733–744. doi: 10.4049/jimmunol.1901121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäättelä M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31(4):261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- Kasioumi P, Vrazeli P, Vezyraki P, Zerikiotis S, Katsouras C, Damalas A, Angelidis C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int J Oncol. 2019;54(3):821–832. doi: 10.3892/ijo.2018.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschinski DM. On heat and cells and proteins. Physiology. 2004;19(1):11–15. doi: 10.1152/nips.01403.2002. [DOI] [PubMed] [Google Scholar]
- Kim MY, Oglesbee M. Virus-heat shock protein interaction and a novel axis for innate antiviral immunity. Cells. 2012;1(3):646–666. doi: 10.3390/cells1030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris AC, Theodoropoulos GE, Aroni K, Saetta A, Davaris PS. Immunohistochemical expression of C-myc oncogene, heat shock protein 70 and HLA-DR molecules in malignant cutaneous melanoma. Virchows Arch. 1995;426(5):461–467. doi: 10.1007/BF00193169. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43(3):168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MJ, Costa C, Eixarch H, Tepavcevic V, Castillo M, Martin R, Lubetzki C, Aigrot MS, Montalban X, Espejo C (2014) Hsp70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS One 9 (8):e105737 [DOI] [PMC free article] [PubMed]
- Mansilla MJ, Montalban X, Espejo C. Heat shock protein 70: roles in multiple sclerosis. Mol Med. 2012;18(1):1018–1028. doi: 10.2119/molmed.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menares E, Gálvez-Cancino F, Cáceres-Morgado P, Ghorani E, López E, Díaz X, Saavedra-Almarza J, Figueroa DA, Roa E, Quezada SA, Lladser A. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10(1):4401. doi: 10.1038/s41467-019-12319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Measel JW, Amoss MS, Rao A, Greene JF. Immunophenotypic characterization of tumor infiltrating lymphocytes and peripheral blood lymphocytes isolated from melanomatous and non-melanomatous Sinclair miniature swine. Vet Immunol Immunopathol. 1996;55(1):189–203. doi: 10.1016/S0165-2427(96)05621-8. [DOI] [PubMed] [Google Scholar]
- Morrison BJ, Roman JA, Luke TC, Nagabhushana N, Raviprakash K, Williams M, Sun P. Antibody-dependent NK cell degranulation as a marker for assessing antibody-dependent cytotoxicity against pandemic 2009 influenza A(H1N1) infection in human plasma and influenza-vaccinated transchromosomic bovine intravenous immunoglobulin therapy. J Virol Methods. 2017;248:7–18. doi: 10.1016/j.jviromet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25(1):88–98. doi: 10.1111/j.1755-148X.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, Alamiri A, Hughes T, Bines SD, Kaufman HL, Overbeck A, Mehrotra S, Hernandez C, Nishimura MI, Guevara-Patino JA, Le Poole IC (2013) Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med 5 (174):174ra128 [DOI] [PMC free article] [PubMed]
- Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6(4):337–344. doi: 10.1379/1466-1268(2001)006<0337:AMHPSN>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmervoll B, Chtcheglova LA, Juhasz K, Cremades N, Aprile FA, Sonnleitner A, Hinterdorfer P, Vigh L, Preiner J, Balogi Z. Cell surface localised Hsp70 is a cancer specific regulator of clathrin-independent endocytosis. FEBS Letters. 2015;589(19PartB):2747–2753. doi: 10.1016/j.febslet.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169(10):5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, Wilmott JS, Scolyer RA, Tüting T, Palendira U, Gyorki D, Mueller SN, Huntington ND, Bedoui S, Hölzel M, Mackay LK, Waithman J, Gebhardt T. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature. 2019;565(7739):366–371. doi: 10.1038/s41586-018-0812-9. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34(32):4153–4161. doi: 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov M, Huile G, Multhoff G. Membrane heat shock protein 70: a theranostic target for cancer therapy. Philosophical Transactions of the Royal Society b: Biological Sciences. 2018;373(1738):20160526. doi: 10.1098/rstb.2016.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers L, Coupe N, Payne M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology (Oxford) 2019;58(Suppl 7):vii7–vii16. doi: 10.1093/rheumatology/kez418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunother Cancer. 2014;2(1):29. doi: 10.1186/s40425-014-0029-x. [DOI] [Google Scholar]
- Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178(4):1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Perry CJ, Meeth K, Thakral D, Damsky W, Micevic G, Kaech S, Blenman K, Bosenberg M (2017) UV-induced somatic mutations elicit a functional T cell response in the YUMMER1.7 mouse melanoma model. Pigment Cell Melanoma Res 30 (4):428–435. 10.1111/pcmr.12591 [DOI] [PMC free article] [PubMed]
- Witt SN. Hsp70 molecular chaperones and Parkinson’s disease. Biopolymers. 2010;93(3):218–228. doi: 10.1002/bip.21302. [DOI] [PubMed] [Google Scholar]
- Yang N-S, Sun WH, McCabe D. Developing particle-mediated gene-transfer technology for research into gene therapy of cancer. Mol Med Today. 1996;2(11):476–481. doi: 10.1016/1357-4310(96)10046-0. [DOI] [PubMed] [Google Scholar]
- Young RA. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang Y, Lindstrom AR, Lin TY, Lam KS, Li Y. Peptide-Based Materials for Cancer Immunotherapy. Theranostics. 2019;9(25):7807–7825. doi: 10.7150/thno.37194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.